Abstract
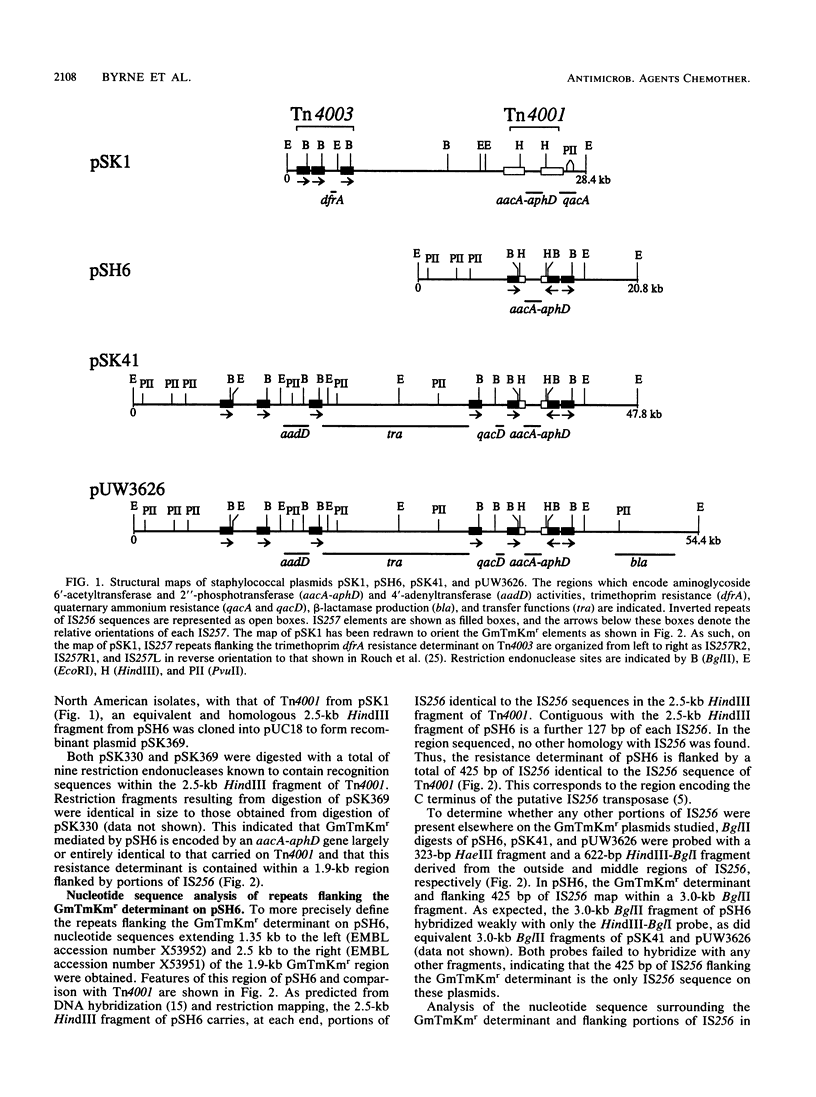
Plasmid-encoded resistance to the aminoglycosides gentamicin (Gm), tobramycin (Tm), and kanamycin (Km) (GmTmKmr) in strains of Staphylococcus aureus isolated in Australia and North America appears to be mediated by one resistance determinant. In Australian isolates, this determinant is flanked by inverted copies of a 1.3-kb insertion sequence, IS256, thereby forming a composite transposon, Tn4001. Analysis of two conjugative plasmids and a related nonconjugative plasmid from strains of S. aureus isolated in North America showed that the GmTmKmr determinant on these plasmids is also flanked by inverted repeats. In the nonconjugative plasmid, these repeats include only 425 bp of IS256 immediately adjacent to the GmTmKmr region and identical to that on Tn4001. This truncated Tn4001 element is flanked by copies of the insertion element IS257, and together these elements form a truncated Tn4001-IS257 hybrid transposonlike structure. A third copy of IS257 was located 418 bp from the hybrid structure. The truncated Tn4001 and three repeats of IS257 were present at a conserved site on the plasmids studied. Four additional copies of IS257 were identified on the two conjugative plasmids. These elements flank determinants for resistance to the aminoglycosides neomycin and paromomycin and to ethidium bromide and quaternary ammonium compounds, as well as the region involved in conjugative plasmid transfer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Coughter J. P., Johnston J. L. Plasmid-encoded trimethoprim resistance in staphylococci. Antimicrob Agents Chemother. 1986 May;29(5):733–740. doi: 10.1128/aac.29.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983 Jul;24(1):70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch D. K., Goering R. V., Ruff E. A. Isolation and preliminary characterization of a plasmid mutant derepressed for conjugal transfer in Staphylococcus aureus. Plasmid. 1984 Nov;12(3):197–202. doi: 10.1016/0147-619x(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Barberis-Maino L., Berger-Bächi B., Weber H., Beck W. D., Kayser F. H. IS431, a staphylococcal insertion sequence-like element related to IS26 from Proteus vulgaris. Gene. 1987;59(1):107–113. doi: 10.1016/0378-1119(87)90271-x. [DOI] [PubMed] [Google Scholar]
- Byrne M. E., Rouch D. A., Skurray R. A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989 Sep 30;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- Delecluse A., Bourgouin C., Klier A., Rapoport G. Nucleotide sequence and characterization of a new insertion element, IS240, from Bacillus thuringiensis israelensis. Plasmid. 1989 Jan;21(1):71–78. doi: 10.1016/0147-619x(89)90088-7. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M. T., Lyon B. R., Messerotti L. J., Skurray R. A. Chromosome- and plasmid-mediated gentamicin resistance in Staphylococcus aureus encoded by Tn4001. J Med Microbiol. 1987 Sep;24(2):139–144. doi: 10.1099/00222615-24-2-139. [DOI] [PubMed] [Google Scholar]
- Gillespie M. T., Lyon B. R., Skurray R. A. Structural and evolutionary relationships of beta-lactamase transposons from Staphylococcus aureus. J Gen Microbiol. 1988 Nov;134(11):2857–2866. doi: 10.1099/00221287-134-11-2857. [DOI] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. S., Huang R. T., Davies J. Aminocyclitol resistance in Staphylococcus aureus: presence of plasmids and aminocyclitol-modifying enzymes. Plasmid. 1983 Mar;9(2):147–158. doi: 10.1016/0147-619x(83)90017-3. [DOI] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Mobilization of the gentamicin resistance gene in Enterococcus faecalis. Antimicrob Agents Chemother. 1990 Jun;34(6):1278–1280. doi: 10.1128/aac.34.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H. W., Sweeney H. M., Weinstein R. A., Kabins S. A., Nathan C., Cohen S. Structural and phenotypic varieties of gentamicin resistance plasmids in hospital strains of Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1982 May;21(5):773–779. doi: 10.1128/aac.21.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Gillespie M. T., Byrne M. E., May J. W., Skurray R. A. Plasmid-mediated resistance to gentamicin in Staphylococcus aureus: the involvement of a transposon. J Med Microbiol. 1987 Mar;23(2):101–110. doi: 10.1099/00222615-23-2-101. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Analysis of plasmids in nosocomial strains of multiple-antibiotic-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Jun;23(6):817–826. doi: 10.1128/aac.23.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193(3):554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Timm J., Rauzier J., Gomez-Lus R., Davies J., Gicquel B. Transposition of an antibiotic resistance element in mycobacteria. Nature. 1990 Jun 21;345(6277):739–743. doi: 10.1038/345739a0. [DOI] [PubMed] [Google Scholar]
- Matthews P. R., Reed K. C., Stewart P. R. The cloning of chromosomal DNA associated with methicillin and other resistances in Staphylococcus aureus. J Gen Microbiol. 1987 Jul;133(7):1919–1929. doi: 10.1099/00221287-133-7-1919. [DOI] [PubMed] [Google Scholar]
- McDonnell R. W., Sweeney H. M., Cohen S. Conjugational transfer of gentamicin resistance plasmids intra- and interspecifically in Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1983 Jan;23(1):151–160. doi: 10.1128/aac.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Skurray R. Cloning and polypeptide analysis of the leading region in F plasmid DNA transfer. Plasmid. 1983 May;9(3):262–272. doi: 10.1016/0147-619x(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Rouch D. A., Byrne M. E., Kong Y. C., Skurray R. A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987 Nov;133(11):3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- Rouch D. A., Messerotti L. J., Loo L. S., Jackson C. A., Skurray R. A. Trimethoprim resistance transposon Tn4003 from Staphylococcus aureus encodes genes for a dihydrofolate reductase and thymidylate synthetase flanked by three copies of IS257. Mol Microbiol. 1989 Feb;3(2):161–175. doi: 10.1111/j.1365-2958.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- Rouch D. A., Skurray R. A. IS257 from Staphylococcus aureus: member of an insertion sequence superfamily prevalent among gram-positive and gram-negative bacteria. Gene. 1989;76(2):195–205. doi: 10.1016/0378-1119(89)90160-1. [DOI] [PubMed] [Google Scholar]
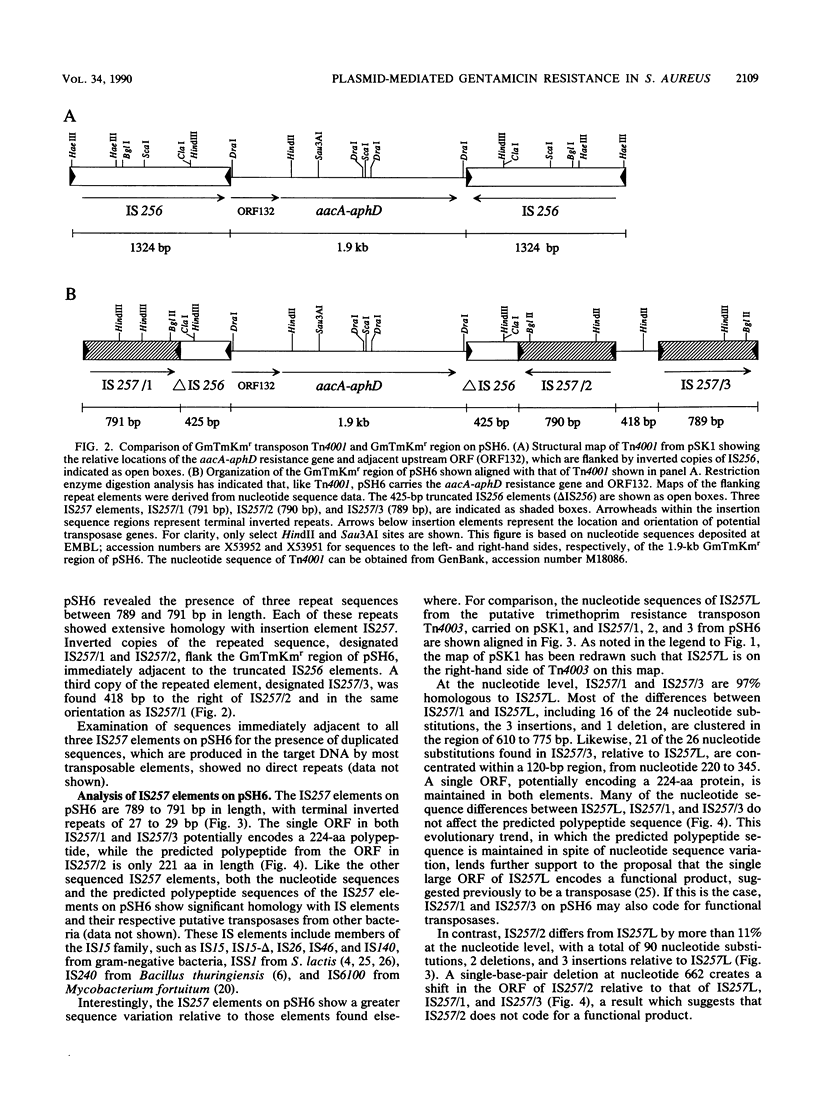
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurray R. A., Rouch D. A., Lyon B. R., Gillespie M. T., Tennent J. M., Byrne M. E., Messerotti L. J., May J. W. Multiresistant Staphylococcus aureus: genetics and evolution of epidemic Australian strains. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):19–39. doi: 10.1093/jac/21.suppl_c.19. [DOI] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrs M. J., Courvalin P., Foster T. J. Genetic analysis of gentamicin resistance in methicillin- and gentamicin-resistant strains of Staphylococcus aureus isolated in Dublin hospitals. Antimicrob Agents Chemother. 1988 Aug;32(8):1174–1181. doi: 10.1128/aac.32.8.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennent J. M., Lyon B. R., Gillespie M. T., May J. W., Skurray R. A. Cloning and expression of Staphylococcus aureus plasmid-mediated quaternary ammonium resistance in Escherichia coli. Antimicrob Agents Chemother. 1985 Jan;27(1):79–83. doi: 10.1128/aac.27.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennent J. M., Lyon B. R., Midgley M., Jones I. G., Purewal A. S., Skurray R. A. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J Gen Microbiol. 1989 Jan;135(1):1–10. doi: 10.1099/00221287-135-1-1. [DOI] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Identification and cloning of the conjugative transfer region of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1989 Feb;171(2):684–691. doi: 10.1128/jb.171.2.684-691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Mobility of gentamicin resistance genes from staphylococci isolated in the United States: identification of Tn4031, a gentamicin resistance transposon from Staphylococcus epidermidis. Antimicrob Agents Chemother. 1989 Aug;33(8):1335–1341. doi: 10.1128/aac.33.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Bolton S., Bradley J., Duckworth G., Moorhouse E. C., Grubb W. B. The international spread of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1987 Jan;9(1):60–71. doi: 10.1016/0195-6701(87)90097-1. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]