Abstract
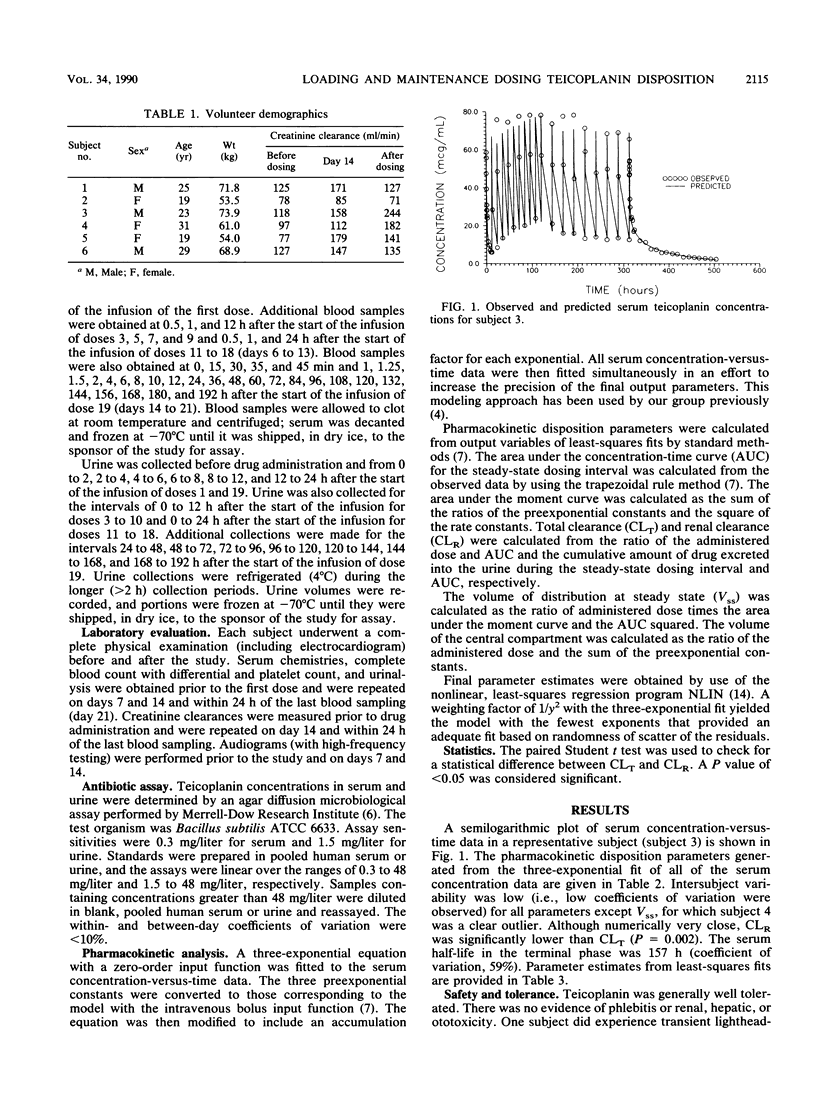
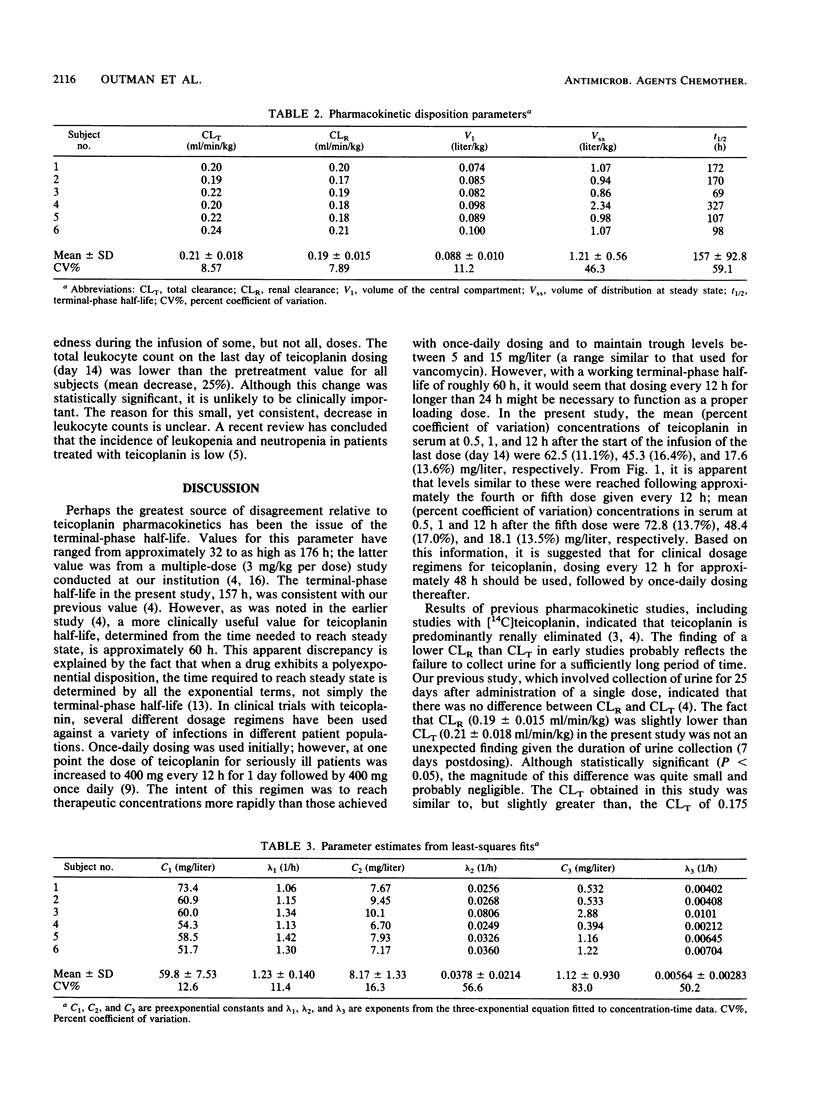
Teicoplanin is an investigational glycopeptide antibiotic that is structurally and microbiologically similar to vancomycin. Since teicoplanin possesses a very long elimination half-life, the manufacturer suggests that the drug be administered every 12 h for the first day of therapy and once daily thereafter. We studied the multiple-dose (6 mg/kg per dose) pharmacokinetics of teicoplanin in volunteers following intravenous administration every 12 h for 5 days and then every 24 h for 9 days in an attempt to identify the optimal duration of the every-12-h loading-dose regimen. Multiple serum samples were obtained throughout the study, including intensive sampling after the first and last doses; urine was collected during the entire study. A three-exponential equation was fitted to the serum concentration data. The mean terminal-phase half-life was 157 +/- 93 h. Concentrations of teicoplanin in serum similar to those observed after the administration of the last dose (day 14) were observed following the fourth or fifth dose given every 12 h. Therefore, it is suggested that for clinical dosing regimens for teicoplanin, dosing every 12 h for approximately 48 h should be used, followed by once-daily dosing thereafter.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borghi A., Coronelli C., Faniuolo L., Allievi G., Pallanza R., Gallo G. G. Teichomycins, new antibiotics from Actinoplanes teichomyceticus nov. sp. IV. Separation and characterization of the components of teichomycin (teicoplanin). J Antibiot (Tokyo) 1984 Jun;37(6):615–620. doi: 10.7164/antibiotics.37.615. [DOI] [PubMed] [Google Scholar]
- Buniva G., Del Favero A., Bernareggi A., Patoia L., Palumbo R. Pharmacokinetics of 14C-teicoplanin in healthy volunteers. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):23–28. doi: 10.1093/jac/21.suppl_a.23. [DOI] [PubMed] [Google Scholar]
- Carver P. L., Nightingale C. H., Quintiliani R., Sweeney K., Stevens R. C., Maderazo E. Pharmacokinetics of single- and multiple-dose teicoplanin in healthy volunteers. Antimicrob Agents Chemother. 1989 Jan;33(1):82–86. doi: 10.1128/aac.33.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Favero A., Patoia L., Bucaneve G., Biscarini L., Menichetti F. Leukopenia with neutropenia associated with teicoplanin therapy. DICP. 1989 Jan;23(1):45–47. doi: 10.1177/106002808902300109. [DOI] [PubMed] [Google Scholar]
- Erickson R. C., Hildebrand A. R., Hoffman P. F., Gibson C. B. A sensitive bioassay for teicoplanin in serum in the presence or absence of other antibiotics. Diagn Microbiol Infect Dis. 1989 May-Jun;12(3):235–241. doi: 10.1016/0732-8893(89)90020-5. [DOI] [PubMed] [Google Scholar]
- Greenwood D. Microbiological properties of teicoplanin. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):1–13. doi: 10.1093/jac/21.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Leport C., Perronne C., Massip P., Canton P., Leclercq P., Bernard E., Lutun P., Garaud J. J., Vilde J. L. Evaluation of teicoplanin for treatment of endocarditis caused by gram-positive cocci in 20 patients. Antimicrob Agents Chemother. 1989 Jun;33(6):871–876. doi: 10.1128/aac.33.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P., Garaud J. J., Parenti F. A multicentre open clinical trial of teicoplanin in infections caused by gram-positive bacteria. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):61–67. doi: 10.1093/jac/21.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- Martino P., Venditti M., Micozzi A., Brandimarte C., Gentile G., Santini C., Serra P. Teicoplanin in the treatment of gram-positive-bacterial endocarditis. Antimicrob Agents Chemother. 1989 Aug;33(8):1329–1334. doi: 10.1128/aac.33.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkin D. H., Mico B. A., Sitrin R. D., Nisbet L. J. Charge and lipophilicity govern the pharmacokinetics of glycopeptide antibiotics. Antimicrob Agents Chemother. 1986 Mar;29(3):440–444. doi: 10.1128/aac.29.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma S., Gastaldo L., Corti A. Teicoplanin, a new antibiotic from Actinoplanes teichomyceticus nov. sp. Antimicrob Agents Chemother. 1984 Dec;26(6):917–923. doi: 10.1128/aac.26.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina G. L., Bonati M. Pharmacokinetics of teicoplanin in man after intravenous administration. J Pharmacokinet Biopharm. 1984 Apr;12(2):119–128. doi: 10.1007/BF01059273. [DOI] [PubMed] [Google Scholar]
- Tsukayama D. T., Guay D. R., Gustilo R. B., Wicklund B., Gruninger R., Peterson P. K. The effect of anaerobiosis on antistaphylococcal antibiotics. Orthopedics. 1988 Sep;11(9):1285–1289. doi: 10.3928/0147-7447-19880901-10. [DOI] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]



