Abstract
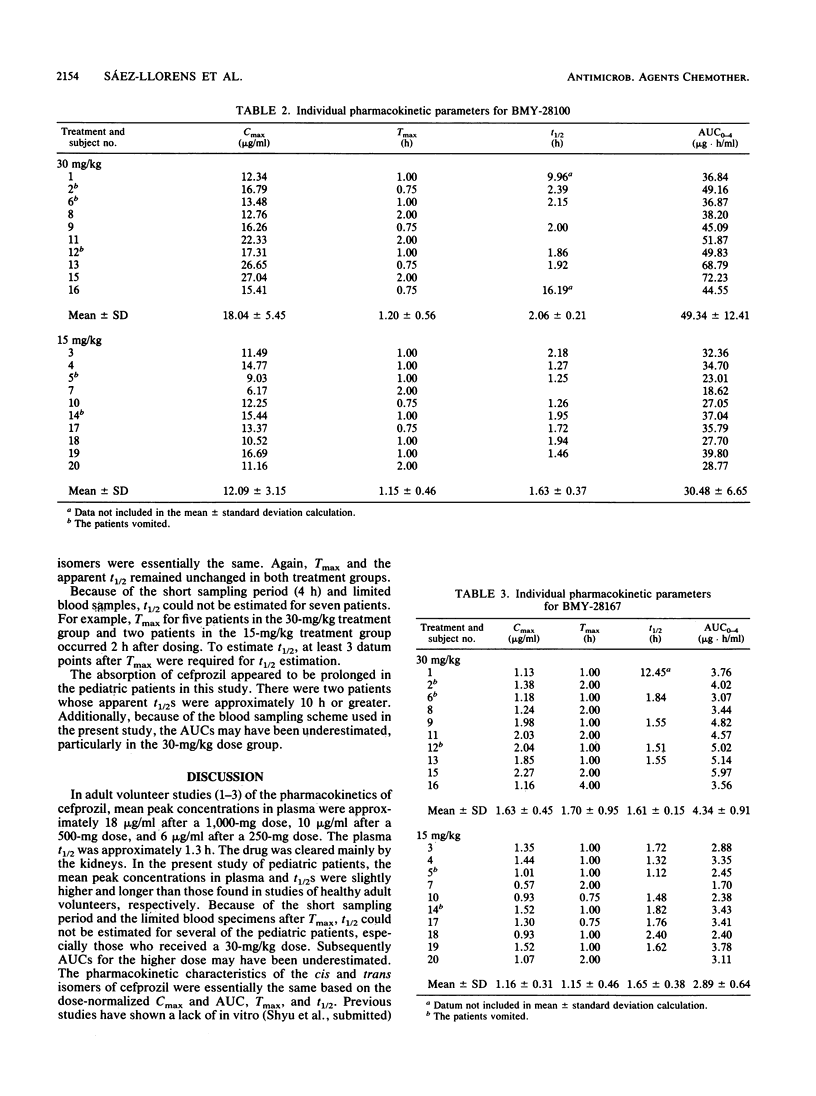
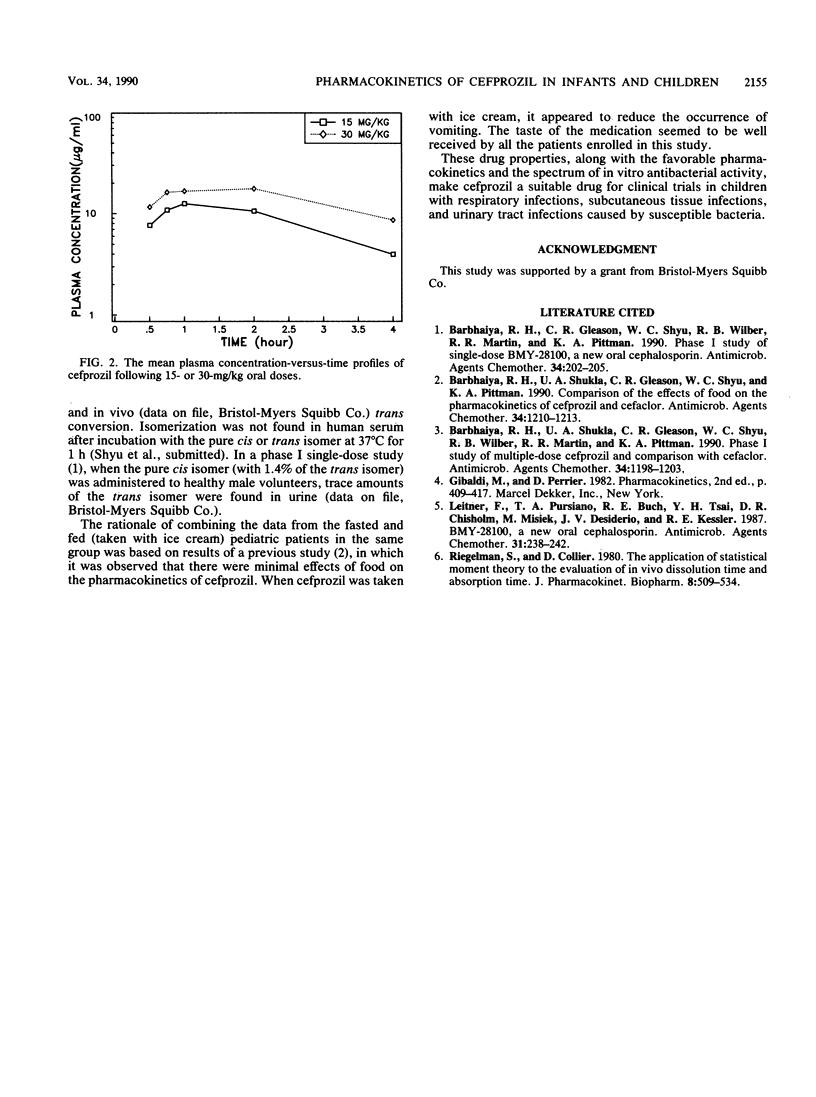
Twenty pediatric patients (ages, between 8 months and 8 years) received a single oral dose of cefprozil at levels of 15 and 30 mg/kg of body weight. Cefprozil consists of cis (BMY-28100) and trans (BMY-28167) isomers in an approximately 90:10 ratio. Six plasma samples were collected from each pediatric patient and assayed for drug concentrations. As measured by a microbiological assay, peak concentrations of 11.16 and 15.93 micrograms of cefprozil per ml occurred at 1 h for patients who received the 15- and 30-mg/kg doses, respectively. The respective mean half-lives of cefprozil were 1.77 and 2.14 h, and the respective mean areas under the curve were 28.05 and 45.28 micrograms.h/ml for patients who received the 15- and 30-mg/kg doses. When measured by a high-pressure liquid chromatography method, peak concentrations of 12.09 and 18.04 micrograms of the cis isomer per ml were obtained at 1 h, with mean half-lives of 1.63 and 2.06 h and mean areas under the curve of 30.48 and 49.34 micrograms.h/ml in patients who received the 15- and 30-mg/kg doses, respectively. For the trans isomer, peak concentrations of 1.16 and 1.63 micrograms/ml occurred at 1 h, respectively, with mean half-lives of 1.61 and 1.65 h and mean areas under the curve of 2.89 and 4.34 micrograms.h/ml in patients who received the 15- and 30-mg/kg doses, respectively.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Gleason C. R., Shyu W. C., Wilber R. B., Martin R. R., Pittman K. A. Phase I study of single-dose BMY-28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1990 Feb;34(2):202–205. doi: 10.1128/aac.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Pittman K. A. Comparison of the effects of food on the pharmacokinetics of cefprozil and cefaclor. Antimicrob Agents Chemother. 1990 Jun;34(6):1210–1213. doi: 10.1128/aac.34.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Shukla U. A., Gleason C. R., Shyu W. C., Wilber R. B., Martin R. R., Pittman K. A. Phase I study of multiple-dose cefprozil and comparison with cefaclor. Antimicrob Agents Chemother. 1990 Jun;34(6):1198–1203. doi: 10.1128/aac.34.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner F., Pursiano T. A., Buck R. E., Tsai Y. H., Chisholm D. R., Misiek M., Desiderio J. V., Kessler R. E. BMY 28100, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Feb;31(2):238–243. doi: 10.1128/aac.31.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegelman S., Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980 Oct;8(5):509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]



