Abstract
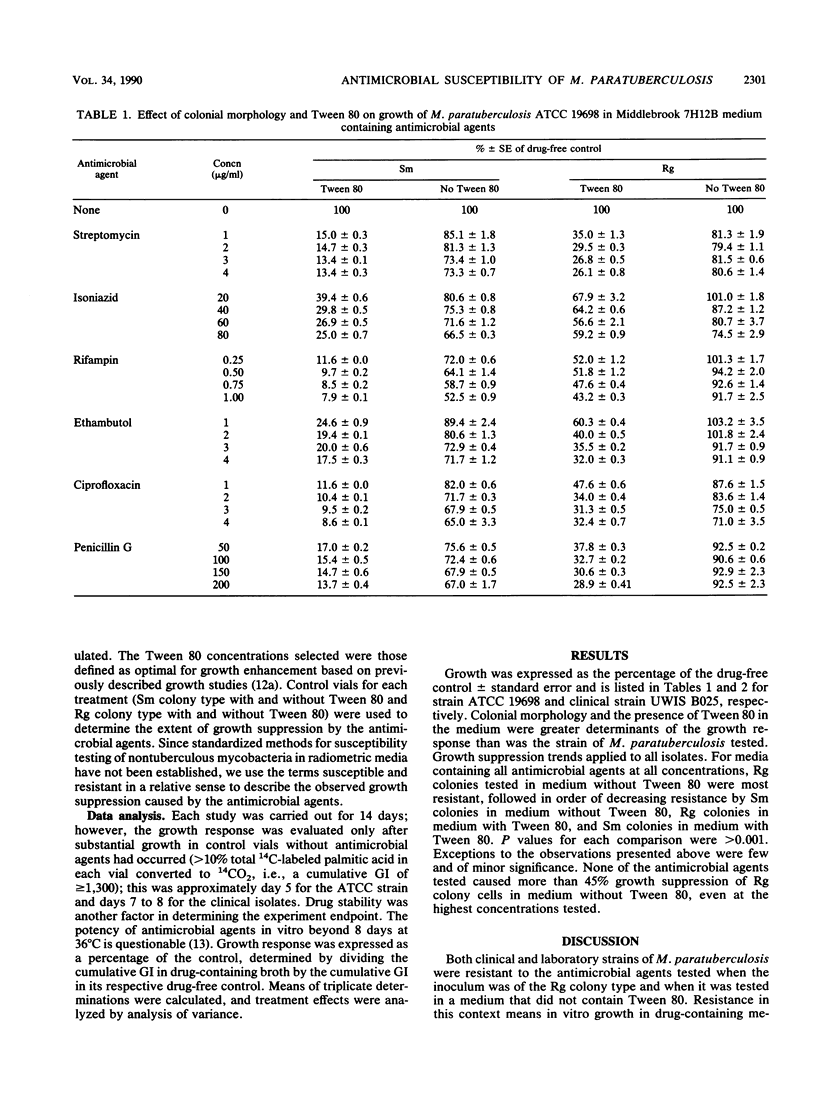
Smooth (Sm) and rough (Rg) colonial types of Mycobacterium paratuberculosis ATCC 19698 and two clinical isolates were tested to examine their growth responses in medium containing antimicrobial agents. Susceptibility tests were done in Middlebrook 7H12B medium with and without Tween 80 and one of the following antimicrobial agents: streptomycin, isoniazid, rifampin, ethambutol, ciprofloxacin, and penicillin G. Growth responses in the presence of antimicrobial agents led to the following observations. (i) In the absence of Tween, Rg colony types were more resistant than Sm colony types; (ii) the addition of Tween 80 significantly increased the susceptibility of both Sm and Rg colony types; however, the increase was greater with the Sm colony types. These studies showed that the antimicrobial susceptibility of M. paratuberculosis was significantly affected when Tween 80 was present in either the primary culture medium or the drug susceptibility test medium. In the absence of the perturbing influence of Tween 80, M. paratuberculosis was resistant to the antimicrobial agents tested.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow W. W., Brennan P. J. Isolation in high frequency of rough variants of Mycobacterium intracellulare lacking C-mycoside glycopeptidolipid antigens. J Bacteriol. 1982 Apr;150(1):381–384. doi: 10.1128/jb.150.1.381-384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J. Biochemical characteristics of various strains of Mycobacterium paratuberculosis. Am J Vet Res. 1986 Jul;47(7):1442–1445. [PubMed] [Google Scholar]
- Collins M. T., Kenefick K. B., Sockett D. C., Lambrecht R. S., McDonald J., Jorgensen J. B. Enhanced radiometric detection of Mycobacterium paratuberculosis by using filter-concentrated bovine fecal specimens. J Clin Microbiol. 1990 Nov;28(11):2514–2519. doi: 10.1128/jcm.28.11.2514-2519.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. L., Rastogi N., Clavel-Sérès S., Clément F., Thorel M. F. Structure of the cell envelope of Mycobacterium avium. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Apr;264(1-2):49–66. doi: 10.1016/s0176-6724(87)80124-4. [DOI] [PubMed] [Google Scholar]
- Hui J., Gordon N., Kajioka R. Permeability barrier to rifampin in mycobacteria. Antimicrob Agents Chemother. 1977 May;11(5):773–779. doi: 10.1128/aac.11.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajioka R., Hui J. The pleiotropic effect of spontaneous single-step variant production in Mycobacterium intracellulare. Scand J Respir Dis. 1978 Apr;59(2):91–100. [PubMed] [Google Scholar]
- Lambrecht R. S., Carriere J. F., Collins M. T. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl Environ Microbiol. 1988 Apr;54(4):910–916. doi: 10.1128/aem.54.4.910-916.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Fukunaga M., Taniguchi H. Plasmid deoxyribonucleic acid and translucent-to-opaque variation in Mycobacterium intracellulare 103. J Bacteriol. 1981 May;146(2):656–659. doi: 10.1128/jb.146.2.656-659.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Ogawa M., Udou T. Morphological changes induced by beta-lactam antibiotics in Mycobacterium avium-intracellulare complex. Antimicrob Agents Chemother. 1985 Apr;27(4):541–547. doi: 10.1128/aac.27.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S. P., Samsonoff W. A., Ruck R. E. Effects of surface-active agents on drug susceptibility levels and ultrastructure of Mycobacterium avium complex organisms isolated from AIDS patients. Diagn Microbiol Infect Dis. 1988 Sep;11(1):11–19. doi: 10.1016/0732-8893(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Potar M. C., David H. L. Action of antituberculous and beta-lactam drugs (including imipenem) against extra- and intra-cellularly growing Mycobacterium avium-intracellulare. Ann Inst Pasteur Microbiol. 1988 Mar-Apr;139(2):225–232. doi: 10.1016/0769-2609(88)90007-5. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Lewis C. W., Jr Effect of oleic acid on growth and cell structure of mycobacteria. J Bacteriol. 1965 Nov;90(5):1438–1447. doi: 10.1128/jb.90.5.1438-1447.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Broth microdilution testing of susceptibilities to 30 antimicrobial agents of Mycobacterium avium strains from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1987 Oct;31(10):1579–1584. doi: 10.1128/aac.31.10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel R. M., Lambrecht R. S., Collins M. T. Effect of polyoxyethylene sorbate compounds (Tweens) on colonial morphology, growth, and ultrastructure of Mycobacterium paratuberculosis. APMIS. 1990 Oct;98(10):901–908. doi: 10.1111/j.1699-0463.1990.tb05013.x. [DOI] [PubMed] [Google Scholar]


