Abstract
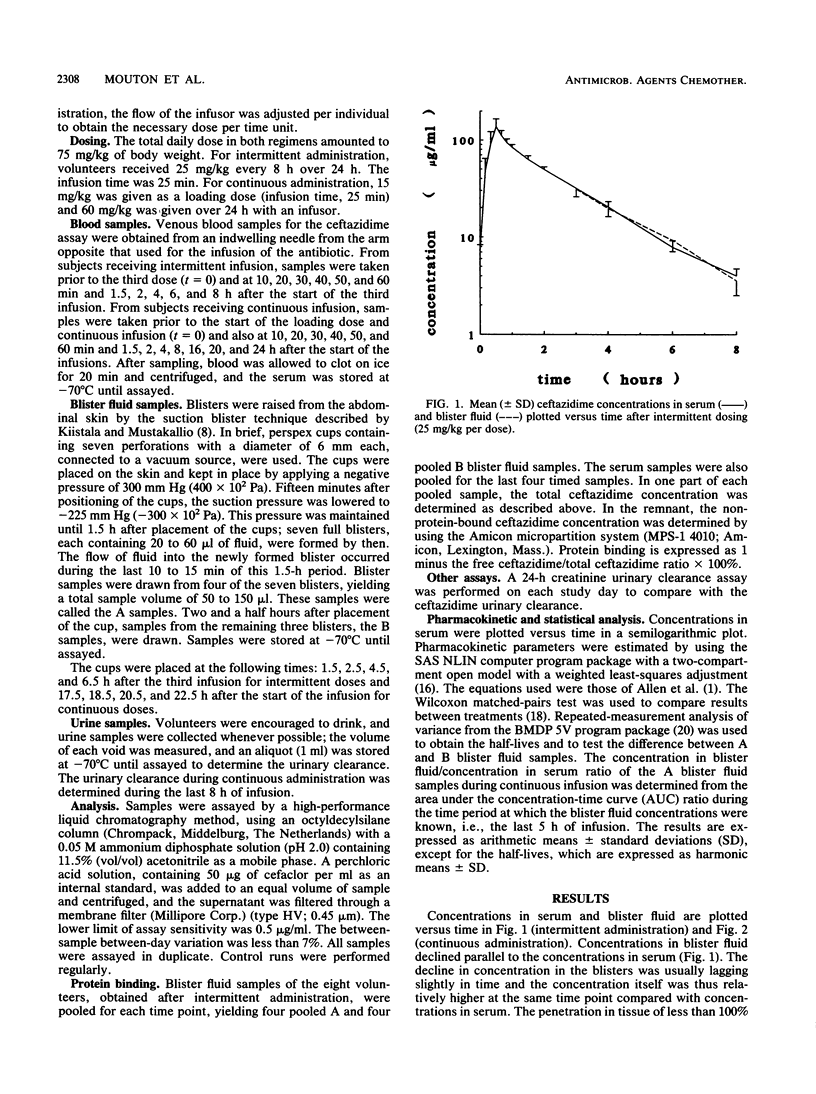
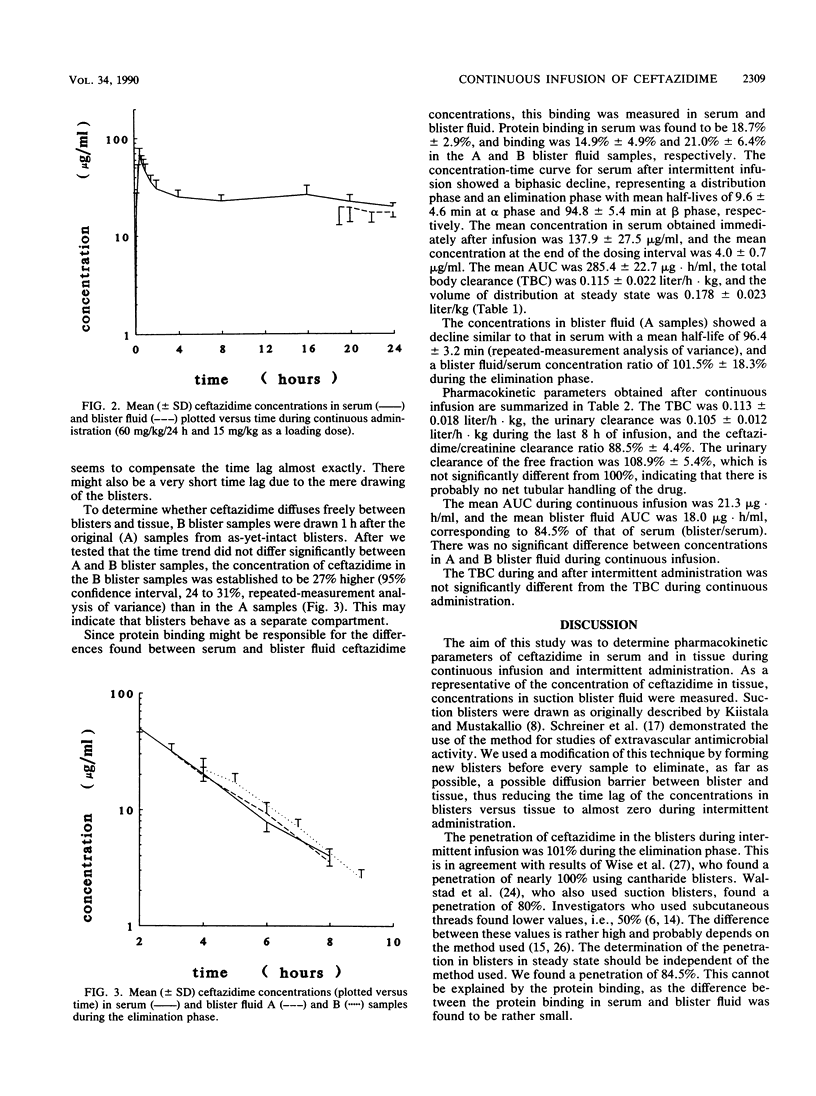
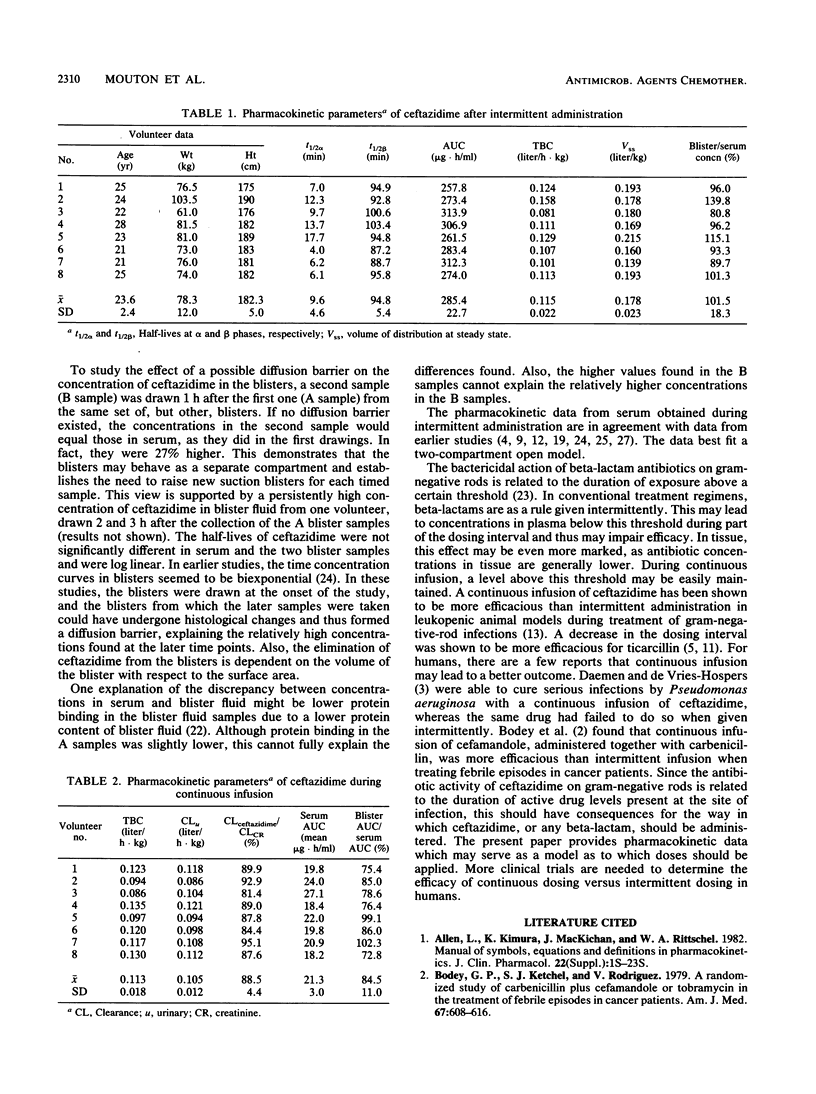
The pharmacokinetics of ceftazidime were investigated during intermittent (II) and continuous (CI) infusion in eight healthy male volunteers in a crossover fashion. The total daily dose was 75 mg/kg of body weight per 24 h in both regimens, given in three doses of 25 mg/kg/8 h (II) or 60 mg/kg/24 h with 15 mg/kg as a loading dose (CI). After the third dose (II) and during CI, serum and blister fluid samples were taken. Seven new blisters were raised for each timed sample by a suction blister technique. Blisters took 90 min to form. Samples were then taken from four blisters (A samples) and 1 h later were taken from the remaining three (B samples). The concentration of ceftazidime was determined using a high-performance liquid chromatography method. After II, the concentrations in serum immediately after infusion (t = 30 min) and 8 h after the start of the infusion were 137.9 (standard deviation [SD], 27.5) and 4.0 (SD, 0.7) micrograms/ml, respectively. The half-life at alpha phase (t1/2 alpha) was 9.6 min (SD, 4.6), t1/2 beta was 94.8 min (SD, 5.4), area under the concentration-time curve (AUC) was 285.4 micrograms.h/ml (SD, 22.7), total body clearance was 0.115 liter/h.kg (SD, 0.022), and volume of distribution at steady state was 0.178 liter/kg (SD, 0.023). The blister fluid (A) samples showed a decline in concentration parallel to that of the concentrations in serum during the elimination phase with a ratio of 1:1. The t1/2 of the A samples was 96.4 min (SD, 3.2). The concentration of ceftazidime in the B blister fluid samples was significantly higher (27%) than in the A samples over time. This shows that blisters may behave as a separate compartment and establishes the need to raise new blisters for each timed sample. The mean AUC/h during continuous infusion was 21.3 micrograms . h/ml (SD, 3.0). The total body clearance was 0.113 liter/h . kg (SD, 0.018), the urinary clearance was 0.105 liter/h . kg (SD, 0.012), and the ceftazidime/creatinine clearance ratio was 0.885. The mean AUC of blister fluid per hour was 84.5% (18.0 micrograms . h/liter; SD, 3.6) compared with that of serum. The A samples did not differ significantly from the B samples. The implications of continuous infusion of beta-lactams for treatment of serious infections are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P., Ketchel S. J., Rodriguez V. A randomized study of carbenicillin plus cefamandole or tobramycin in the treatment of febrile episodes in cancer patients. Am J Med. 1979 Oct;67(4):608–616. doi: 10.1016/0002-9343(79)90242-0. [DOI] [PubMed] [Google Scholar]
- Daenen S., de Vries-Hospers H. Cure of Pseudomonas aeruginosa infection in neutropenic patients by continuous infusion of ceftazidime. Lancet. 1988 Apr 23;1(8591):937–937. doi: 10.1016/s0140-6736(88)91741-2. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Gorski S., Person A., Mangura C., Chmel H. Clindamycin elimination in patients with liver disease. J Antimicrob Chemother. 1981 Oct;8(4):277–281. doi: 10.1093/jac/8.4.277. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Craig W. A., Brugger H. P., Feller C., Vastola A. P., Brandel J. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J Infect Dis. 1983 May;147(5):910–917. doi: 10.1093/infdis/147.5.910. [DOI] [PubMed] [Google Scholar]
- Horrevorts A. M. In vitro activity of new antimicrobial agents against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Eur J Clin Microbiol. 1987 Jun;6(3):323–324. doi: 10.1007/BF02017629. [DOI] [PubMed] [Google Scholar]
- Karhunen M., Koskela O., Hällström K. Single dose of tinidazole in prophylaxis in infections following vaginal surgery. J Antimicrob Chemother. 1981 Oct;8(4):283–290. doi: 10.1093/jac/8.4.283. [DOI] [PubMed] [Google Scholar]
- Langer M., Mascheroni D., Marcolin R., Gattinoni L. The prone position in ARDS patients. A clinical study. Chest. 1988 Jul;94(1):103–107. doi: 10.1378/chest.94.1.103. [DOI] [PubMed] [Google Scholar]
- LeBel M., Barbeau G., Vallee F., Bergeron M. G. Pharmacokinetics of ceftazidime in elderly volunteers. Antimicrob Agents Chemother. 1985 Nov;28(5):713–715. doi: 10.1128/aac.28.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel M., Spino M. Pulse dosing versus continuous infusion of antibiotics. Pharmacokinetic-pharmacodynamic considerations. Clin Pharmacokinet. 1988 Feb;14(2):71–95. doi: 10.2165/00003088-198814020-00002. [DOI] [PubMed] [Google Scholar]
- Manual of symbols, equations & definitions in pharmacokinetics. J Clin Pharmacol. 1982 Jul;22(7):1S–23S. [PubMed] [Google Scholar]
- Mordenti J. J., Quintiliani R., Nightingale C. H. Combination antibiotic therapy: comparison of constant infusion and intermittent bolus dosing in an experimental animal model. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):313–321. doi: 10.1093/jac/15.suppl_a.313. [DOI] [PubMed] [Google Scholar]
- Naber K. G., Kees F., Grobecker H. Ceftazidime: pharmacokinetics in young volunteers versus elderly patients and therapeutic efficacy with complicated urinary tract infections. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):41–45. doi: 10.1093/jac/12.suppl_a.41. [DOI] [PubMed] [Google Scholar]
- Roosendaal R., Bakker-Woudenberg I. A., van den Berghe-van Raffe M., Vink-van den Berg J. C., Michel B. M. Impact of the dosage schedule on the efficacy of ceftazidime, gentamicin and ciprofloxacin in Klebsiella pneumoniae pneumonia and septicemia in leukopenic rats. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):878–887. doi: 10.1007/BF01963774. [DOI] [PubMed] [Google Scholar]
- Ryan D. M., Hodges B., Spencer G. R., Harding S. M. Simultaneous comparison of three methods for assessing ceftazidime penetration into extravascular fluid. Antimicrob Agents Chemother. 1982 Dec;22(6):995–998. doi: 10.1128/aac.22.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner A., Hellum K. B., Digranes A., Bergman I. Transfer of penicillin G and ampicillin into human skin blisters induced by suction. Scand J Infect Dis Suppl. 1978;(14):233–237. [PubMed] [Google Scholar]
- Sommers D. K., Walters L., Van Wyk M., Harding S. M., Paton A. M., Ayrton J. Pharmacokinetics of ceftazidime in male and female volunteers. Antimicrob Agents Chemother. 1983 Jun;23(6):892–896. doi: 10.1128/aac.23.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandramaga T. B., Van Hecken A., Mullie A., Verbesselt R., De Schepper P. J., Verbist L. Comparative pharmacokinetics of ceftazidime and moxalactam. Antimicrob Agents Chemother. 1982 Aug;22(2):237–241. doi: 10.1128/aac.22.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer B. J., Reman F. C., van Gent C. M. The determination of lipids and proteins in suction blister fluid. J Invest Dermatol. 1979 Oct;73(4):303–305. doi: 10.1111/1523-1747.ep12531833. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Craig W. A. Kinetics of antimicrobial activity. J Pediatr. 1986 May;108(5 Pt 2):835–840. doi: 10.1016/s0022-3476(86)80754-5. [DOI] [PubMed] [Google Scholar]
- Walstad R. A., Hellum K. B., Blika S., Dale L. G., Fredriksen T., Myhre K. I., Spencer G. R. Pharmacokinetics and tissue penetration of ceftazidime: studies on lymph, aqueous humour, skin blister, cerebrospinal and pleural fluid. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):275–282. doi: 10.1093/jac/12.suppl_a.275. [DOI] [PubMed] [Google Scholar]
- Warns H., Lode H., Harnoss C. M., Kemmerich B., Koeppe P., Wagner J. Multiple dose pharmacokinetics and therapeutic results with ceftazidime. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):235–240. doi: 10.1093/jac/12.suppl_a.235. [DOI] [PubMed] [Google Scholar]


