Abstract
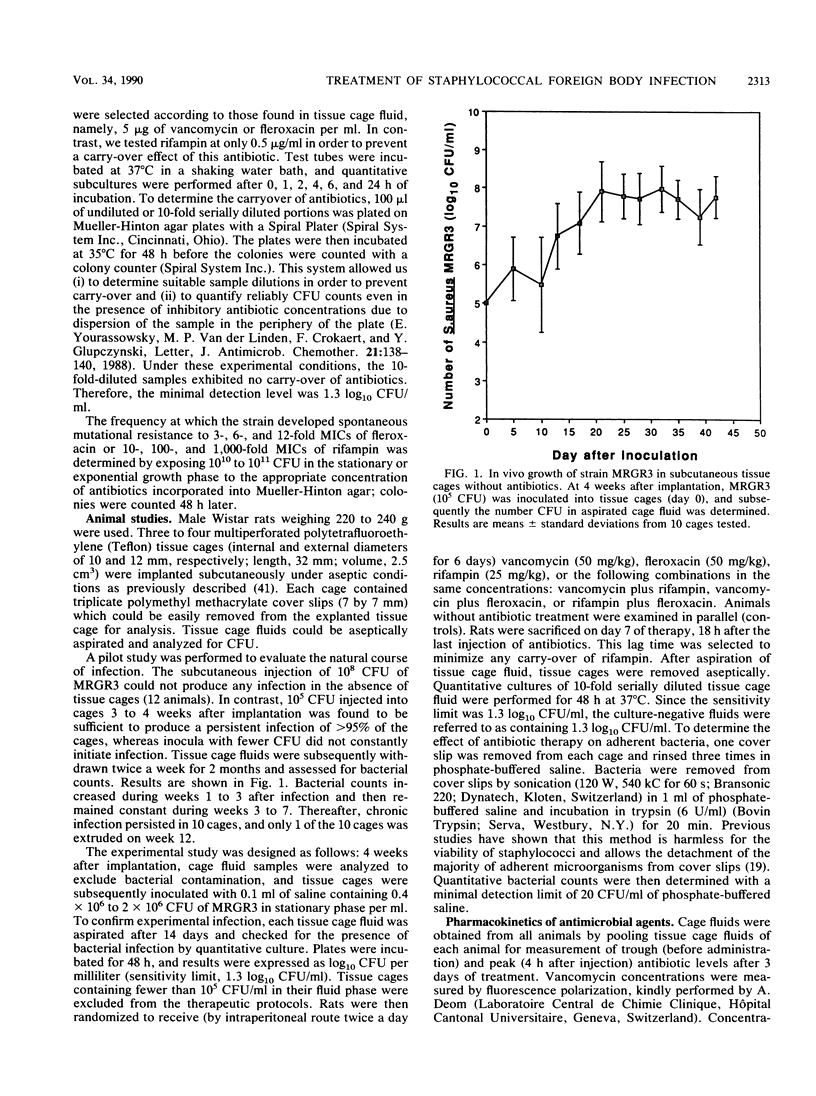
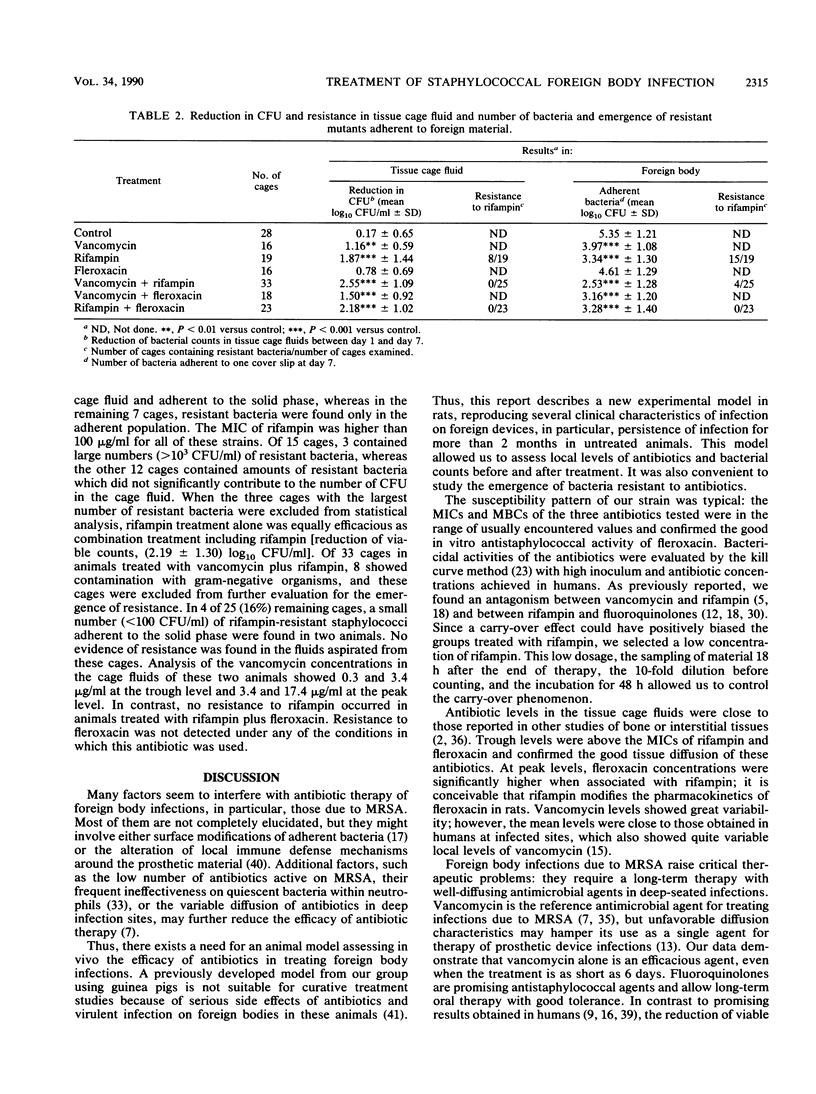
A novel model of experimental foreign body infection was developed in rats: four perforated Teflon tissue cages per animal were implanted subcutaneously and 3 to 4 weeks later were infected with 0.5 x 10(5) to 2 x 10(5) CFU of methicillin-resistant Staphylococcus aureus. After 2 weeks, the number of CFU in the cage fluid was determined [day 1 mean, (7.25 +/- 0.79) log10 CFU/ml], and treatment with vancomycin (50 mg/kg twice a day [BID]), fleroxacin (50 mg/kg BID), or fifampin (25 mg/kg BID), alone and in combination, was initiated for a duration of 6 days. Concentrations of antibiotics in cage fluids were in the range of those encountered in clinical conditions. Eighteen hours after the last injection (day 7), the number of CFU in the cage fluid was determined and the difference between day 1 and day 7 values was calculated. Rifampin, alone and in combination with fleroxacin or vancomycin, was the most effective regimen in reducing the bacterial counts in the tissue cage fluids [(1.87 +/- 1.44, 2.18 +/- 1.02, and 2.55 +/- 1.09 log10) CFU/ml, P less than 0.001, respectively]. After treatment, cage fluids and cages were analyzed for resistant bacteria. Resistance to rifampin occurred in 15 of 19 cages in animals treated with rifampin alone and in 4 of 25 in animals treated with rifampin plus vancomycin. We detected no development of resistance to rifampin in animals treated with rifampin plus fleroxacin or to fleroxacin in animals treated with this antimicrobial agent. In conclusion, regimens including rifampin alone or in combination with vancomycin or fleroxacin were an effective treatment of foreign body infection due to methicillin-resistant S. aureus in reducing bacteria counts, but rifampin monotherapy was compromised by significant emergence of resistance. The combined therapy of fleroxacin with rifampin prevent development of resistance to rifampin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acar J. F., Goldstein F. W., Duval J. Use of rifampin for the treatment of serious staphylococcal and gram-negative bacillary infections. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S502–S506. doi: 10.1093/clinids/5.supplement_3.s502. [DOI] [PubMed] [Google Scholar]
- Acocella G. Pharmacokinetics and metabolism of rifampin in humans. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S428–S432. doi: 10.1093/clinids/5.supplement_3.s428. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Lam K. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis. 1985 Jan;151(1):157–165. doi: 10.1093/infdis/151.1.157. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Morrison J. O. Disparity between timed-kill and checkerboard methods for determination of in vitro bactericidal interactions of vancomycin plus rifampin versus methicillin-susceptible and -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1984 Aug;26(2):220–223. doi: 10.1128/aac.26.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Swinski L. A., Waternaux C. M., Karchmer A. W., Buckley M. J. Risk factors for the development of prosthetic valve endocarditis. Circulation. 1985 Jul;72(1):31–37. doi: 10.1161/01.cir.72.1.31. [DOI] [PubMed] [Google Scholar]
- Chambers H. F. Methicillin-resistant staphylococci. Clin Microbiol Rev. 1988 Apr;1(2):173–186. doi: 10.1128/cmr.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplaces N., Acar J. F. New quinolones in the treatment of joint and bone infections. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S179–S183. doi: 10.1093/clinids/10.supplement_1.s179. [DOI] [PubMed] [Google Scholar]
- Dworkin R. J., Lee B. L., Sande M. A., Chambers H. F. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989 Nov 4;2(8671):1071–1073. doi: 10.1016/s0140-6736(89)91083-0. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Smith S. M., Tillem M., Cherubin C. Rifampin resistance. Development during the therapy of methicillin-resistant Staphylococcus aureus infection. Arch Intern Med. 1985 Jan;145(1):146–148. doi: 10.1001/archinte.145.1.146. [DOI] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L. In vitro antistaphylococcal activity of pefloxacin alone and in combination with other antistaphylococcal drugs. Antimicrob Agents Chemother. 1987 Oct;31(10):1457–1460. doi: 10.1128/aac.31.10.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faville R. J., Jr, Zaske D. E., Kaplan E. L., Crossley K., Sabath L. D., Quie P. G. Staphylococcus aureus endocarditis. Combined therapy with vancomycin and rifampin. JAMA. 1978 Oct 27;240(18):1963–1965. doi: 10.1001/jama.240.18.1963. [DOI] [PubMed] [Google Scholar]
- Garaud J. J., Regnier B., Lassoued K., Pangon B., Leport C. Traitement des staphylococcies graves. Echecs de la rifampicine en association. Quatre observations. Presse Med. 1985 May 4;14(18):1013–1016. [PubMed] [Google Scholar]
- Graziani A. L., Lawson L. A., Gibson G. A., Steinberg M. A., MacGregor R. R. Vancomycin concentrations in infected and noninfected human bone. Antimicrob Agents Chemother. 1988 Sep;32(9):1320–1322. doi: 10.1128/aac.32.9.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N., Kennedy D. J., Reilly P. M., Luppen K. L., Weinandt W. J., Bollinger M. R., Aguirre F., Kodesch F., Saeed A. M. Treatment of bone, joint, and soft-tissue infections with oral ciprofloxacin. Antimicrob Agents Chemother. 1987 Feb;31(2):151–155. doi: 10.1128/aac.31.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristina A. G., Costerton J. W. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985 Feb;67(2):264–273. [PubMed] [Google Scholar]
- Hackbarth C. J., Chambers H. F., Sande M. A. Serum bactericidal activity of rifampin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):611–613. doi: 10.1128/aac.29.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann M., Jaconi M. E., Dahlgren C., Waldvogel F. A., Stendahl O., Lew D. P. Neutrophil bactericidal activity against Staphylococcus aureus adherent on biological surfaces. Surface-bound extracellular matrix proteins activate intracellular killing by oxygen-dependent and -independent mechanisms. J Clin Invest. 1990 Sep;86(3):942–951. doi: 10.1172/JCI114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Barriere S. L., Albrecht L. M., Rybak M. J. Ciprofloxacin and rifampin, alone and in combination, for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1989 Aug;33(8):1184–1187. doi: 10.1128/aac.33.8.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusnik J. E., Parenti F., Sande M. A. The use of rifampicin in staphylococcal infections--a review. J Antimicrob Chemother. 1984 Jun;13 (Suppl 100):61–66. doi: 10.1093/jac/13.suppl_c.61. [DOI] [PubMed] [Google Scholar]
- Karchmer A. W., Archer G. L., Dismukes W. E. Rifampin treatment of prosthetic valve endocarditis due to Staphylococcus epidermidis. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S543–S548. doi: 10.1093/clinids/5.supplement_3.s543. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Moorman D. R. Treatment of experimental staphylococcal infections: effect of rifampin alone and in combination on development of rifampin resistance. Antimicrob Agents Chemother. 1980 Apr;17(4):658–662. doi: 10.1128/aac.17.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. The antimicrobial activity of rifampin: emphasis on the relation to phagocytes. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S463–S467. doi: 10.1093/clinids/5.supplement_3.s463. [DOI] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989 Mar 11;1(8637):537–540. doi: 10.1016/s0140-6736(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Maple P., Hamilton-Miller J., Brumfitt W. Ciprofloxacin resistance in methicillin- and gentamicin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1989 Jul;8(7):622–624. doi: 10.1007/BF01968141. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Bacterial resistance to fluoroquinolones. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S57–S63. doi: 10.1093/clinids/10.supplement_1.s57. [DOI] [PubMed] [Google Scholar]
- Norden C. W., Shaffer M. Treatment of experimental chronic osteomyelitis due to staphylococcus aureus with vancomycin and rifampin. J Infect Dis. 1983 Feb;147(2):352–357. doi: 10.1093/infdis/147.2.352. [DOI] [PubMed] [Google Scholar]
- Piercy E. A., Barbaro D., Luby J. P., Mackowiak P. A. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 1989 Jan;33(1):128–130. doi: 10.1128/aac.33.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E., TOMPSETT R. The survival of staphylococci within human leukocytes. J Exp Med. 1952 Feb;95(2):209–230. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G. L., Smith R. H., Sande M. A. Emergence of rifampin-resistant strains of Staphylococcus aureus during combination therapy with vancomycin and rifampin: a report of two cases. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S507–S508. doi: 10.1093/clinids/5.supplement_3.s507. [DOI] [PubMed] [Google Scholar]
- Sorgel F., Metz R., Naber K., Seelmann R., Muth P. Pharmacokinetics and body fluid penetration of fleroxacin in healthy volunteers. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):155–167. doi: 10.1093/jac/22.supplement_d.155. [DOI] [PubMed] [Google Scholar]
- Sorrell T. C., Packham D. R., Shanker S., Foldes M., Munro R. Vancomycin therapy for methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):344–350. doi: 10.7326/0003-4819-97-3-344. [DOI] [PubMed] [Google Scholar]
- Tshefu K., Zimmerli W., Waldvogel F. A. Short-term administration of rifampin in the prevention or eradication of infection due to foreign bodies. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S474–S480. doi: 10.1093/clinids/5.supplement_3.s474. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Meunier-Carpentier F., Klastersky J. Clinical study of combination therapy with oxacillin and rifampin for staphylococcal infections. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S515–S522. doi: 10.1093/clinids/5.supplement_3.s515. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Lew P. D., Waldvogel F. A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984 Apr;73(4):1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]


