Abstract
AIM—To investigate the longitudinal changes of interstitial and airways disease in resolving chronic lung disease of prematurity (CLD). METHODS—Thirty three infants were studied between 35 and 40 weeks of postconceptional age, and then at three monthly intervals throughout their first year. Measurements of mean arterial oxygen saturation (MSaO2) and its variability (δMSaO2) were recorded. PaCO2 and PaO2 were determined while the infants breathed steady state 50% oxygen via a hood. From these, the alveolar arterial difference (A-a) Do250 was calculated. Airway disease was assessed by the measurement of partial forced expiratory flow volume curves (PEFC) to give V̇max Frc. RESULTS—The cohort mean +/- 95% confidence intervals measured between 35 and 40 weeks were for MSaO2 (89·25 +/- 1·87%, range 75-96·5%) and δMSaO2 (4·79 +/- 0·8%, range 0·16-9·64%), PaCO2 (5·89 +/- 0·56 kpa, range 4·2-10·11 kpa), (A-a) Do250 (22·7 +/- 2·56 kpa, range 6·67-31·4 kpa) and V̇maxFrc (41·5 +/- 8·65 mls/second, range 8·5-103·7 ml/second). The most significant improvement in all measurements occurred within the first three months (P = 0·05). An MSaO2 of less than 90% in room air at 1 year of age was predicted between 35 and 40 weeks postconceptional age by an (A-a) Do250 of greater than 29 kpa, with a sensitivity of 0·85 and a specificity of 0·88, and a PaCO2 greater than 7 kpa predicted a specificity of 0·78 and a sensitivity of 0·88. Predictions were strengthened by combining the above criteria and these then gave a sensitivity and specificity of 1. CONCLUSION—Measurements of (A-a) Do250 and PaCO2 taken between 35 and 40 weeks can be used to assess the degree of pulmonary dysfunction at 1 year. Quantification of the severity of CLD could be used as a measurable end point for early neonatal intervention studies. Keywords: chronic lung disease of prematurity; lung growth; infant pulmonary function.
Full Text
The Full Text of this article is available as a PDF (163.9 KB).
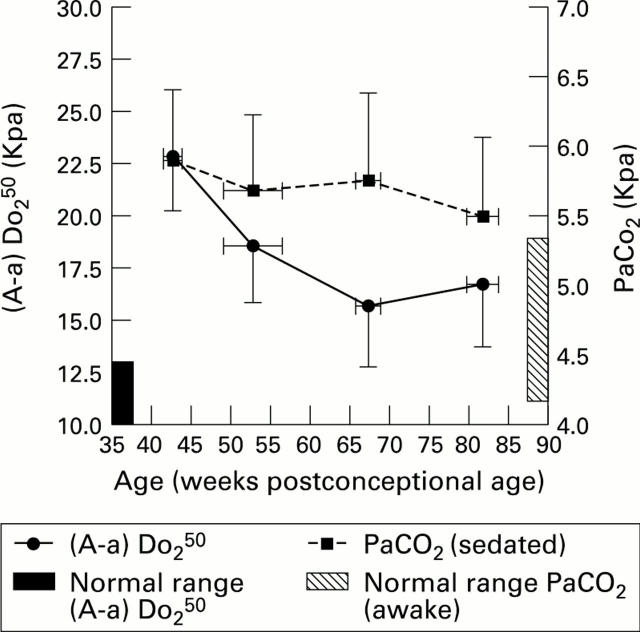
Figure 1 .
Longitudinal changes in (A-a) Do250 and PaCO2 (group means and confidence interval).
Figure 2 .
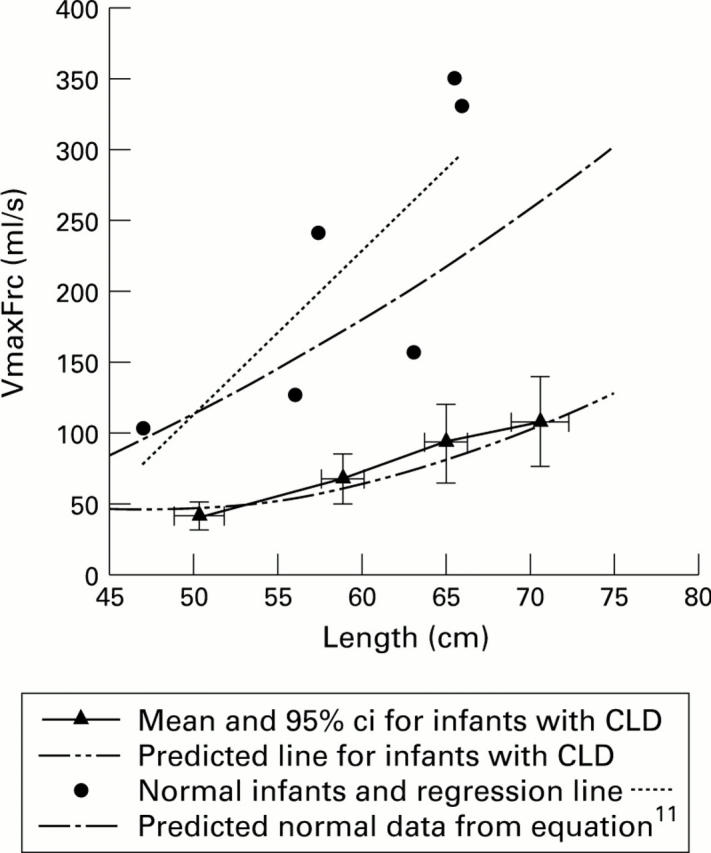
Longitudinal changes in V̇maxFrc.
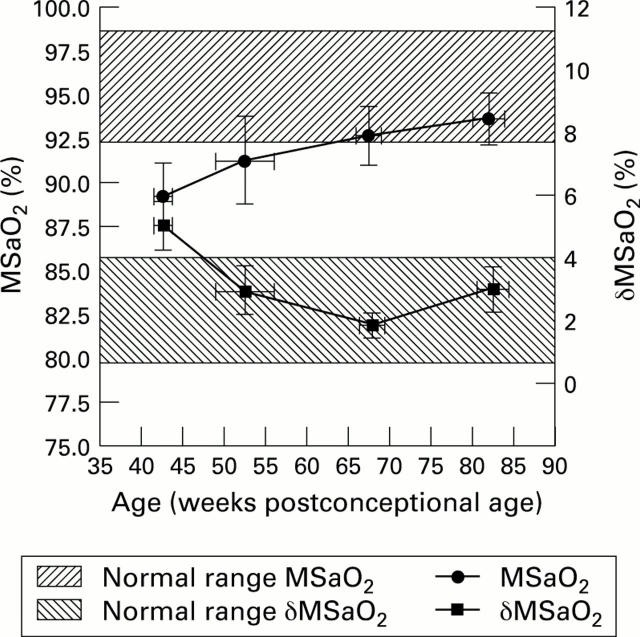
Figure 3 .
Longitudinal changes in MSaO2 and δMSaO2 (cohort means and 95% confidence intervals).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blayney M., Kerem E., Whyte H., O'Brodovich H. Bronchopulmonary dysplasia: improvement in lung function between 7 and 10 years of age. J Pediatr. 1991 Feb;118(2):201–206. doi: 10.1016/s0022-3476(05)80483-4. [DOI] [PubMed] [Google Scholar]
- Bryan M. H., Hardie M. J., Reilly B. J., Swyer P. R. Pulmonary function studies during the first year of life in infants recovering from the respiratory distress syndrome. Pediatrics. 1973 Aug;52(2):169–178. [PubMed] [Google Scholar]
- Calder N. A., Williams B. A., Smyth J., Boon A. W., Kumar P., Hanson M. A. Absence of ventilatory responses to alternating breaths of mild hypoxia and air in infants who have had bronchopulmonary dysplasia: implications for the risk of sudden infant death. Pediatr Res. 1994 Jun;35(6):677–681. doi: 10.1203/00006450-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Chambers H. M., van Velzen D. Ventilator-related pathology in the extremely immature lung. Pathology. 1989 Apr;21(2):79–83. doi: 10.3109/00313028909059539. [DOI] [PubMed] [Google Scholar]
- Chan K. N., Wong Y. C., Silverman M. Relationship between infant lung mechanics and childhood lung function in children of very low birthweight. Pediatr Pulmonol. 1990;8(2):74–81. doi: 10.1002/ppul.1950080204. [DOI] [PubMed] [Google Scholar]
- Chessex P., Bélanger S., Piedboeuf B., Pineault M. Influence of energy substrates on respiratory gas exchange during conventional mechanical ventilation of preterm infants. J Pediatr. 1995 Apr;126(4):619–624. doi: 10.1016/s0022-3476(95)70364-0. [DOI] [PubMed] [Google Scholar]
- Cunningham S., Deere S., Elton R. A., McIntosh N. Neonatal physiological trend monitoring by computer. Int J Clin Monit Comput. 1992 Dec;9(4):221–227. doi: 10.1007/BF01133617. [DOI] [PubMed] [Google Scholar]
- Hansen T. W., Wallach M., Dey A. N., Boivin P., Vohr B., Oh W. Prognostic value of clinical and radiological status on day 28 of life for subsequent course in very low birthweight (< 1,500g) babies with bronchopulmonary dysplasia. Pediatr Pulmonol. 1993 Jun;15(6):327–331. doi: 10.1002/ppul.1950150603. [DOI] [PubMed] [Google Scholar]
- Hislop A. A., Haworth S. G. Airway size and structure in the normal fetal and infant lung and the effect of premature delivery and artificial ventilation. Am Rev Respir Dis. 1989 Dec;140(6):1717–1726. doi: 10.1164/ajrccm/140.6.1717. [DOI] [PubMed] [Google Scholar]
- Hislop A. A., Wigglesworth J. S., Desai R., Aber V. The effects of preterm delivery and mechanical ventilation on human lung growth. Early Hum Dev. 1987 May;15(3):147–164. doi: 10.1016/0378-3782(87)90003-x. [DOI] [PubMed] [Google Scholar]
- Iles R., Edmunds A. T. Prediction of early outcome in resolving chronic lung disease of prematurity after discharge from hospital. Arch Dis Child. 1996 Apr;74(4):304–308. doi: 10.1136/adc.74.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebourges F., Moriette G., Boulé M., Delaperche M. F., Relier J. P., Gaultier C. Pulmonary function in infancy and in childhood following mechanical ventilation in the neonatal period. Pediatr Pulmonol. 1990;9(1):34–40. doi: 10.1002/ppul.1950090108. [DOI] [PubMed] [Google Scholar]
- Levene S., Lear G. H., McKenzie S. A. Comparison of pulse oximeters in healthy sleeping infants. Respir Med. 1989 May;83(3):233–235. doi: 10.1016/s0954-6111(89)80037-x. [DOI] [PubMed] [Google Scholar]
- Mallory G. B., Jr, Chaney H., Mutich R. L., Motoyama E. K. Longitudinal changes in lung function during the first three years of premature infants with moderate to severe bronchopulmonary dysplasia. Pediatr Pulmonol. 1991;11(1):8–14. doi: 10.1002/ppul.1950110103. [DOI] [PubMed] [Google Scholar]
- Margraf L. R., Tomashefski J. F., Jr, Bruce M. C., Dahms B. B. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis. 1991 Feb;143(2):391–400. doi: 10.1164/ajrccm/143.2.391. [DOI] [PubMed] [Google Scholar]
- Moriette G., Gaudebout C., Clement A., Boule M., Bion B., Relier J. P., Gaultier C. Pulmonary function at 1 year of age in survivors of neonatal respiratory distress: a multivariate analysis of factors associated with sequelae. Pediatr Pulmonol. 1987 Jul-Aug;3(4):242–250. doi: 10.1002/ppul.1950030409. [DOI] [PubMed] [Google Scholar]
- Morray J. P., Fox W. W., Kettrick R. G., Downes J. J. Improvement in lung mechanics as a function of age in the infant with severe bronchopulmonary dysplasia. Pediatr Res. 1982 Apr;16(4 Pt 1):290–294. doi: 10.1203/00006450-198204000-00009. [DOI] [PubMed] [Google Scholar]
- Mortensson W., Lindroth M. The course of bronchopulmonary dysplasia. A radiographic follow-up. Acta Radiol Diagn (Stockh) 1986 Jan-Feb;27(1):19–22. doi: 10.1177/028418518602700104. [DOI] [PubMed] [Google Scholar]
- Northway W. H., Jr, Moss R. B., Carlisle K. B., Parker B. R., Popp R. L., Pitlick P. T., Eichler I., Lamm R. L., Brown B. W., Jr Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990 Dec 27;323(26):1793–1799. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- Northway W. H., Jr, Rosan R. C., Porter D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967 Feb 16;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- Pereira G. R., Baumgart S., Bennett M. J., Stallings V. A., Georgieff M. K., Hamosh M., Ellis L. Use of high-fat formula for premature infants with bronchopulmonary dysplasia: metabolic, pulmonary, and nutritional studies. J Pediatr. 1994 Apr;124(4):605–611. doi: 10.1016/s0022-3476(05)83143-9. [DOI] [PubMed] [Google Scholar]
- Samiec T. D., Radmacher P., Hill T., Adamkin D. H. Measured energy expenditure in mechanically ventilated very low birth weight infants. Am J Med Sci. 1994 Mar;307(3):182–184. doi: 10.1097/00000441-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Stocker J. T. Pathologic features of long-standing "healed" bronchopulmonary dysplasia: a study of 28 3- to 40-month-old infants. Hum Pathol. 1986 Sep;17(9):943–961. doi: 10.1016/s0046-8177(86)80646-3. [DOI] [PubMed] [Google Scholar]
- Stocks J., Silverman M. Infant pulmonary function testing workshop II London, England, September 12, 1990. Pediatr Pulmonol. 1991;10(3):219–222. doi: 10.1002/ppul.1950100316. [DOI] [PubMed] [Google Scholar]
- Tager I. B., Ngo L., Hanrahan J. P. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med. 1995 Sep;152(3):977–983. doi: 10.1164/ajrccm.152.3.7663813. [DOI] [PubMed] [Google Scholar]
- Tepper R. S., Morgan W. J., Cota K., Taussig L. M. Expiratory flow limitation in infants with bronchopulmonary dysplasia. J Pediatr. 1986 Dec;109(6):1040–1046. doi: 10.1016/s0022-3476(86)80296-7. [DOI] [PubMed] [Google Scholar]