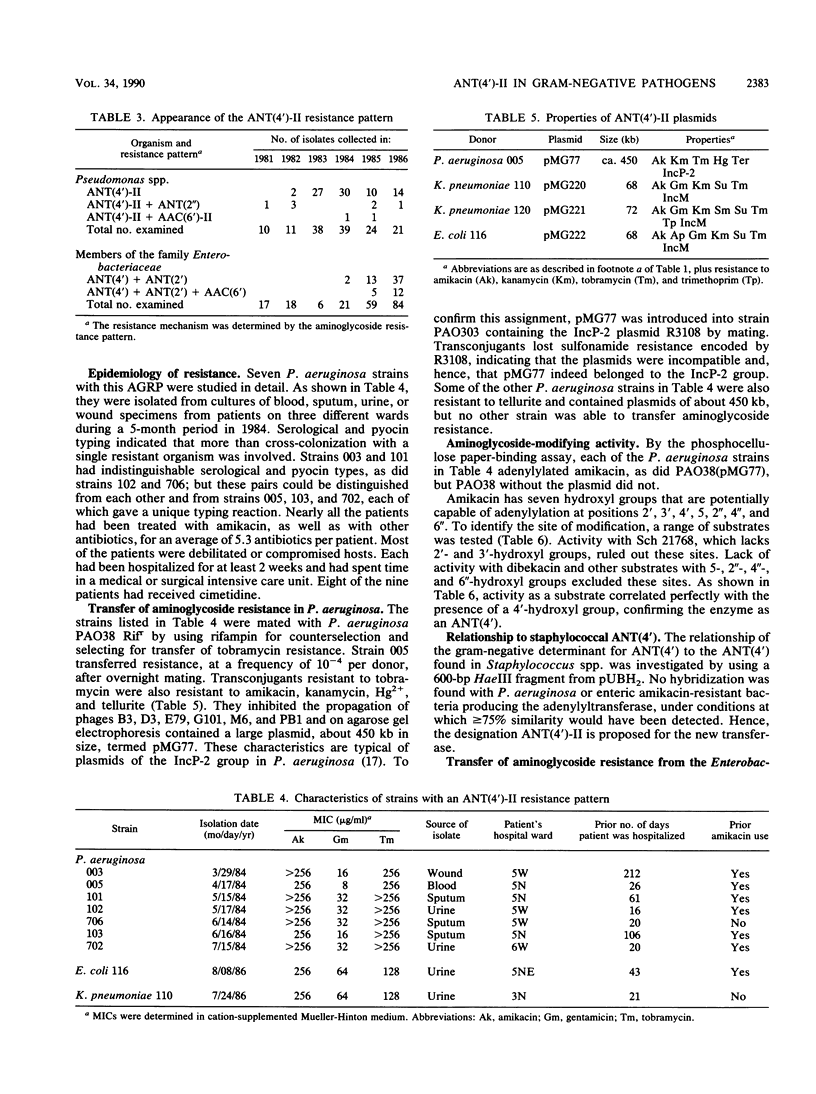
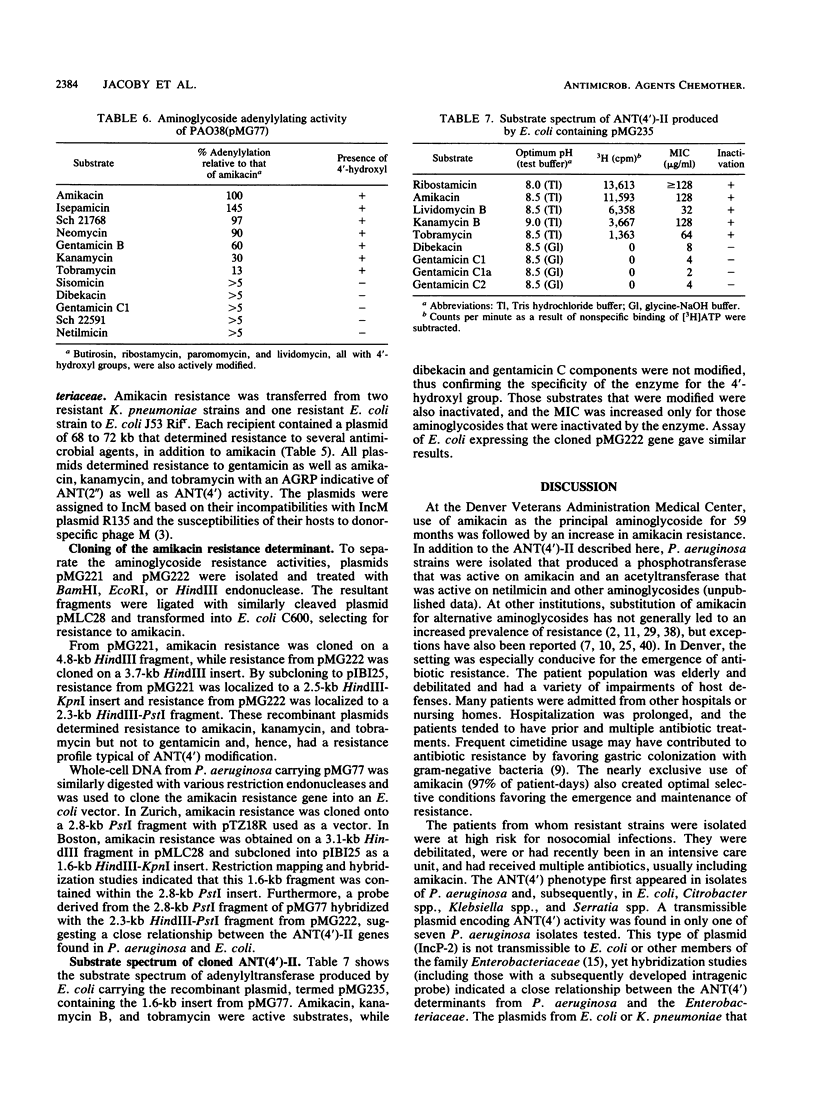
Abstract
Following the use of amikacin as the principal aminoglycoside at a Denver hospital, amikacin resistance appeared first in Pseudomonas aeruginosa and then in Escherichia coli, Klebsiella pneumoniae, and other enteric organisms from debilitated and compromised patients who had spent time in intensive care units and who had been treated with multiple antibiotics, usually including amikacin. In a P. aeruginosa isolate, resistance to amikacin and tobramycin was transferable by the IncP-2 plasmid pMG77, while in E. coli and K. pneumoniae resistance was carried by the transmissible plasmids pMG220, pMG221, and pMG222 belonging to the IncM group. Isolates and transconjugants produced an enzyme with adenyltransferase activity with substrates having a 4'-hydroxyl group, such as amikacin, kanamycin, neomycin, Sch 21768, isepamicin (Sch 21420), or tobramycin, but not with aminoglycosides lacking this target, such as dibekacin, netilmicin, sisomicin, or gentamicin C components. Genes encoding the 4'-aminoglycoside nucleotidyltransferase [ANT(4')] activity were cloned from pMG77, pMG221, and pMG222. A DNA probe prepared from the ANT(4') found in P. aeruginosa hybridized with the ANT(4') determinant found in E. coli. A probe for the ANT(4') from Staphylococcal spp., which differs in its modification of substrates, like dibekacin, that have a 4"- but not a 4'-hydroxyl group, failed to hybridize with the gram-negative ANT(4') determinant, which consequently has been termed ANT(4')-II.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betts R. F., Valenti W. M., Chapman S. W., Chonmaitree T., Mowrer G., Pincus P., Messner M., Robertson R. Five-year surveillance of aminoglycoside usage in a university hospital. Ann Intern Med. 1984 Feb;100(2):219–222. doi: 10.7326/0003-4819-100-2-219. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Bradley D. E., Hedges R. W., Fleming J., Lecatsas G. Bacteriophage M: an incompatibility group M plasmid-specific phage. J Gen Microbiol. 1983 Jul;129(7):2271–2276. doi: 10.1099/00221287-129-7-2271. [DOI] [PubMed] [Google Scholar]
- Coombe R. G., George A. M. New plasmid-mediated aminoglycoside adenylyltransferase of broad substrate range that adenylylates amikacin. Antimicrob Agents Chemother. 1981 Jul;20(1):75–80. doi: 10.1128/aac.20.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe R. G., George A. M. Purification and properties of an aminoglycoside acetyltransferase from Pseudomonas aeruginosa. Biochemistry. 1982 Mar 2;21(5):871–875. doi: 10.1021/bi00534a009. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. S., Opal S., Kopecko D. J. Progressive increase in antibiotic resistance of gram-negative bacterial isolates. Walter Reed Hospital, 1976 to 1980: specific analysis of gentamicin, tobramycin, and amikacin resistance. Arch Intern Med. 1983 Nov;143(11):2075–2080. [PubMed] [Google Scholar]
- Dickgiesser N., Kreiswirth B. N. Determination of aminoglycoside resistance in Staphylococcus aureus by DNA hybridization. Antimicrob Agents Chemother. 1986 May;29(5):930–932. doi: 10.1128/aac.29.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driks M. R., Craven D. E., Celli B. R., Manning M., Burke R. A., Garvin G. M., Kunches L. M., Farber H. W., Wedel S. A., McCabe W. R. Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization. N Engl J Med. 1987 Nov 26;317(22):1376–1382. doi: 10.1056/NEJM198711263172204. [DOI] [PubMed] [Google Scholar]
- Gaynes R., Groisman E., Nelson E., Casadaban M., Lerner S. A. Isolation, characterization, and cloning of a plasmid-borne gene encoding a phosphotransferase that confers high-level amikacin resistance in enteric bacilli. Antimicrob Agents Chemother. 1988 Sep;32(9):1379–1384. doi: 10.1128/aac.32.9.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Larson T. A. Aminoglycoside resistance in gram-negative bacilli during increased amikacin use. Comparison of experience in 14 United States hospitals with experience in the Minneapolis Veterans Administration Medical Center. Am J Med. 1985 Jul 15;79(1A):1–7. doi: 10.1016/0002-9343(85)90184-6. [DOI] [PubMed] [Google Scholar]
- Gillies R. R., Govan J. R. Typing of Pseudomonas pyocyanea by pyocine production. J Pathol Bacteriol. 1966 Apr;91(2):339–345. doi: 10.1002/path.1700910207. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of R plasmids determining gentamicin resistance by acetylation in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Sep;6(3):239–252. doi: 10.1128/aac.6.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of an R plasmid in Pseudomonas aeruginosa producing amikacin (BB-K8), butirosin, kanamycin, tobramycin, and sisomicin resistance. Antimicrob Agents Chemother. 1974 Dec;6(6):807–810. doi: 10.1128/aac.6.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L., Knobel L., Mammen P. Properties of IncP-2 plasmids of Pseudomonas spp. Antimicrob Agents Chemother. 1983 Aug;24(2):168–175. doi: 10.1128/aac.24.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J. F., Jr, McNeill W. F., Price K. E., Kresel P. A. Evidence for a chromosomal site specifying amikacin resistance in multiresistant Serratia marcescens. Antimicrob Agents Chemother. 1982 Apr;21(4):587–591. doi: 10.1128/aac.21.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner M., Macicková T., Krcméry V. Occurrence of 4'-O-aminoglycoside-nucleotidyltransferase in clinical strains of Enterobacteriaceae. Infection. 1989 Mar-Apr;17(2):100–101. doi: 10.1007/BF01646887. [DOI] [PubMed] [Google Scholar]
- Kettner M., Macicková T., Navarová J., Krcméry V., Havlík J. Mechanisms of transferable amikacin resistance in enterobacteria in a Czechoslovak clinic. J Antimicrob Chemother. 1988 Jul;22(1):82–84. doi: 10.1093/jac/22.1.82. [DOI] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988 Jan;32(1):15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic F., Martel A., Capmau M. L., Baca B., Goebel P., Chardon H., Soussy C. J., Duval J., Bouanchaud D. H. New plasmid-mediated nucleotidylation of aminoglycoside antibiotics in Staphlococcus aureus. Antimicrob Agents Chemother. 1976 Aug;10(2):258–264. doi: 10.1128/aac.10.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque R. C., Medeiros A. A., Jacoby G. A. Molecular cloning and DNA homology of plasmid-mediated beta-lactamase genes. Mol Gen Genet. 1987 Feb;206(2):252–258. doi: 10.1007/BF00333581. [DOI] [PubMed] [Google Scholar]
- Levine J. F., Maslow M. J., Leibowitz R. E., Pollock A. A., Hanna B. A., Schaefler S., Simberkoff M. S., Rahal J. J., Jr Amikacin-resistant gram-negative bacilli: correlation of occurrence with amikacin use. J Infect Dis. 1985 Feb;151(2):295–300. doi: 10.1093/infdis/151.2.295. [DOI] [PubMed] [Google Scholar]
- Maloney J., Rimland D., Stephens D. S., Terry P., Whitney A. M. Analysis of amikacin-resistant Pseudomonas aeruginosa developing in patients receiving amikacin. Arch Intern Med. 1989 Mar;149(3):630–634. [PubMed] [Google Scholar]
- Moody M. M., de Jongh C. A., Schimpff S. C., Tillman G. L. Long-term amikacin use. Effects on aminoglycoside susceptibility patterns of gram-negative bacilli. JAMA. 1982 Sep 10;248(10):1199–1202. doi: 10.1001/jama.248.10.1199. [DOI] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. Amikacin resistance associated with a plasmid-borne aminoglycoside phosphotransferase in Escherichia coli. Antimicrob Agents Chemother. 1979 Nov;16(5):598–604. doi: 10.1128/aac.16.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A. M., Paul G. C., Jacoby G. A. Properties of PSE-2 beta-lactamase and genetic basis for its production in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1983 Sep;24(3):362–369. doi: 10.1128/aac.24.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanam P., Kayser F. H. Purification and characterization of an aminoglycoside inactivating enzyme from Staphylococcus epidermidis FK109 that nucleotidylates the 4'- and 4''-hydroxyl groups of the aminoglycoside antibiotics. J Antibiot (Tokyo) 1978 Apr;31(4):343–351. doi: 10.7164/antibiotics.31.343. [DOI] [PubMed] [Google Scholar]
- Santanam P., Kayser F. H. Tobramycin adenylyltransferase: a new aminoglycoside-inactivating enzyme from Staphylococcus epidermidis. J Infect Dis. 1976 Aug;134 (Suppl):S33–S39. doi: 10.1093/infdis/134.supplement_1.s33. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Kumada T., Hsieh W. C., Chung H. Y., Chong Y., Hare R. S., Miller G. H., Sabatelli F. J., Howard J. Comparison of aminoglycoside resistance patterns in Japan, Formosa, and Korea, Chile, and the United States. Antimicrob Agents Chemother. 1985 Aug;28(2):282–288. doi: 10.1128/aac.28.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Collatz E. Heterogeneity of 6'-N-acetyltransferases of type 4 conferring resistance to amikacin and related aminoglycosides in members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1988 Aug;32(8):1289–1291. doi: 10.1128/aac.32.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoof R., Hubrechts J. M., Nyssen H. J., Roebben E. Three-year survey of amikacin use and aminoglycoside resistance in a general hospital in Belgium. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):183–185. doi: 10.1007/BF01963076. [DOI] [PubMed] [Google Scholar]
- WAHBA A. H. HOSPITAL INFECTION WITH PSEUDOMONAS PYOCYANEA: AN INVESTIGATION BY A COMBINED PYOCINE AND SEROLOGICAL TYPING METHOD. Br Med J. 1965 Jan 9;1(5427):86–89. doi: 10.1136/bmj.1.5427.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. J., Sewell C. M., Koza M. A., Clarridge J. E. Antibiotic resistance patterns during aminoglycoside restriction. Am J Med Sci. 1985 Dec;290(6):223–227. doi: 10.1097/00000441-198512000-00001. [DOI] [PubMed] [Google Scholar]


