Abstract
Urinary 5-L-oxoproline was measured in term and preterm infants from shortly after birth until 6 weeks of postnatal age to determine their ability to synthesise glycine. In term infants the excretion was five to 10 times that seen in normal adults, increasing from 105 µmol/mmol creatinine in the first 72 hours after birth to 170 µmol/mmol creatinine at 6 weeks of age. There was a significant inverse linear correlation between the excretion of 5-L-oxoproline and length of gestation or birthweight. By 6 weeks of age there was no longer a significant difference in 5-L-oxoproline between term and preterm infants. There was no difference in the excretion of 5-L-oxoproline between boys and girls, or between infants fed on human milk or an artificial formula. If, in part, variability in the excretion of 5-L-oxoproline is determined by the extent to which the endogenous formation of glycine is adequate, then glycine formation may be marginal during early life, more so in preterm than in term infants, providing additional evidence that glycine is a conditionally essential amino acid in the neonate. Keywords: glycine; γ-glutamyl cycle; protein synthesis; conditionally essential amino acids
Full Text
The Full Text of this article is available as a PDF (139.6 KB).
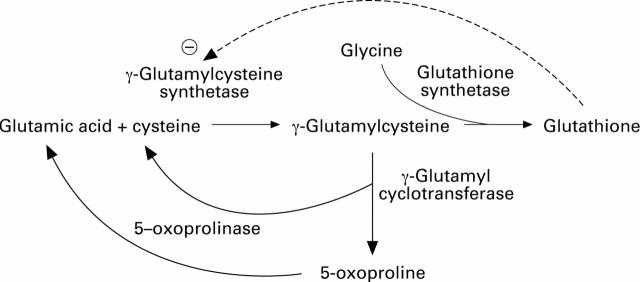
Figure 1 .
Glutathione, γ-glutamylcysteinlglycine is synthesised in two stages catalysed by glutamate-cysteine ligase ( γ-glutamylcysteine synthetase) (EC 6.3.2.2), and glutathione synthase (EC 6.3.2.3). Glutathione inhibits feedback on γ-glutamylcysteinlglycine synthase, so that in the congenital absence of glutathione glutamate excess γ-glutamylcysteinlglycine is cleaved by , γ-glutamyl cyclotransferase (EC 2.3.2.4) to give 5-L-oxoproline, which can be converted to glutamic acid by 5-L-oxoprolinase. Increased urinary 5-L-oxoproline is also produced when the availability of glycine, the substrate for glutathione synthetase, is limited.
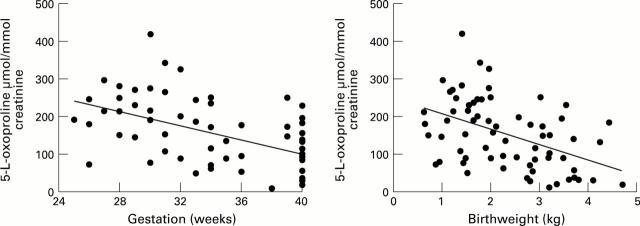
Figure 2 .
The excretion of 5-L-oxoproline/creatinine was measured in the urine of newborn infants within 72 hours of birth. There was a close correlation between birthweight and gestational age, and both showed a strong correlation with the excretion of 5-L-oxoproline (birthweight = 245−[40 ×5-L-oxoproline]; r =−0.46, P = 0.0001: gestational age = 458 −[9×5-L-oxoproline]; r= 0.5, P = 0.00002).
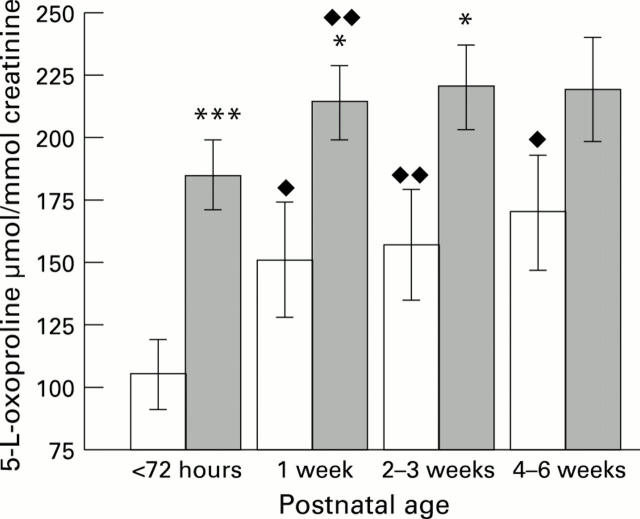
Figure 3 .
The excretion of 5-L-oxoproline/creatinine was measured in the urine of term infants (open bars) and preterm infants (shaded bars) within 72 hours of birth. and at intervals up to 6 weeks of age. Values shown are mean (SE). with significant differences: *P < 0.05; *** P < 0.01 for preterms compared with terms of the same age and P < 0.05; P < 0.01 when compared with excretion of 5-L-oxoproline within 72 hours of birth.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNSTEIN H. R., STANKOVIC V. The effect of certain vitamin deficiencies on glycine biosynthesis. Biochem J. 1956 Feb;62(2):190–198. doi: 10.1042/bj0620190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catzeflis C., Schutz Y., Micheli J. L., Welsch C., Arnaud M. J., Jéquier E. Whole body protein synthesis and energy expenditure in very low birth weight infants. Pediatr Res. 1985 Jul;19(7):679–687. doi: 10.1203/00006450-198507000-00009. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Amino acid nutrition across the placenta. Nutr Rev. 1992 Jan;50(1):13–15. doi: 10.1111/j.1753-4887.1992.tb02455.x. [DOI] [PubMed] [Google Scholar]
- DUSTIN J. P., MOORE S., BIGWOOD E. J. Chromatographic studies on the excretion of amino acids in early infancy. Metabolism. 1955 Jan;4(1):75–79. [PubMed] [Google Scholar]
- Hoffbrand A. V. Folate deficiency in premature infants. Arch Dis Child. 1970 Aug;45(242):441–444. doi: 10.1136/adc.45.242.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. A., Badaloo A. V., Forrester T., Hibbert J. M., Persaud C. Urinary excretion of 5-oxoproline (pyroglutamic aciduria) as an index of glycine insufficiency in normal man. Br J Nutr. 1987 Sep;58(2):207–214. doi: 10.1079/bjn19870088. [DOI] [PubMed] [Google Scholar]
- Jackson A. A., Persaud C., Meakins T. S., Bundy R. Urinary excretion of 5-L-oxoproline (pyroglutamic acid) is increased in normal adults consuming vegetarian or low protein diets. J Nutr. 1996 Nov;126(11):2813–2822. doi: 10.1093/jn/126.11.2813. [DOI] [PubMed] [Google Scholar]
- Jackson A. A., Shaw J. C., Barber A., Golden M. H. Nitrogen metabolism in preterm infants fed human donor breast milk: the possible essentiality of glycine. Pediatr Res. 1981 Nov;15(11):1454–1461. doi: 10.1203/00006450-198111000-00014. [DOI] [PubMed] [Google Scholar]
- Jackson A. A. The glycine story. Eur J Clin Nutr. 1991 Feb;45(2):59–65. [PubMed] [Google Scholar]
- Järvenpä A. L., Rassin D. K., Kuitunen P., Gaull G. E., Räihä N. C. Feeding the low-birth-weight infant. III. Diet influences bile acid metabolism. Pediatrics. 1983 Nov;72(5):677–683. [PubMed] [Google Scholar]
- MACPHERSON H. T., SLATER J. S. gamma-Amino-eta-butyric, aspartic, glutamic and pyrrolidonecarboxylic acid; their determination and occurrence in grass during conservation. Biochem J. 1959 Apr;71(4):654–660. doi: 10.1042/bj0710654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholzer V. G., Wood C. B., Palmer T., Harrison B. M. Increased pyroglutamic acid levels in patients on artificial diets. Clin Chim Acta. 1975 Jul 23;62(2):299–304. doi: 10.1016/0009-8981(75)90240-5. [DOI] [PubMed] [Google Scholar]
- Persaud C., Forrester T., Jackson A. A. Urinary excretion of 5-L-oxoproline (pyroglutamic acid) is increased during recovery from severe childhood malnutrition and responds to supplemental glycine. J Nutr. 1996 Nov;126(11):2823–2830. doi: 10.1093/jn/126.11.2823. [DOI] [PubMed] [Google Scholar]
- Qureshi I. A., Clermont P., Letarte J. The importance of glyoxylate and other glycine precursors in the hepatic and renal conjugation of benzoate in normal and hyperammonemic mice. Can J Physiol Pharmacol. 1989 Nov;67(11):1426–1430. doi: 10.1139/y89-228. [DOI] [PubMed] [Google Scholar]
- Roberts P. M., Arrowsmith D. E., Rau S. M., Monk-Jones M. E. Folate state of premature infants. Arch Dis Child. 1969 Oct;44(237):637–642. doi: 10.1136/adc.44.237.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDERMAN S. E., HOLT L. E., Jr, DANCIS J., ROITMAN E., BOYER A., BALIS M. E. "Unessential" nitrogen: a limiting factor for human growth. J Nutr. 1962 Sep;78:57–72. doi: 10.1093/jn/78.1.57. [DOI] [PubMed] [Google Scholar]
- Shinka T., Inoue Y., Kuhara T., Matsumoto M., Matsumoto I. Benzoylalanine: detection and identification of an alanine conjugate with benzoic acid in hyperammonemic patients treated with sodium benzoate. Clin Chim Acta. 1985 Oct 15;151(3):293–300. doi: 10.1016/0009-8981(85)90092-0. [DOI] [PubMed] [Google Scholar]
- Shojania A. M. Folic acid and vitamin B12 deficiency in pregnancy and in the neonatal period. Clin Perinatol. 1984 Jun;11(2):433–459. [PubMed] [Google Scholar]
- Thompson J. A., Miles B. S., Fennessey P. V. Urinary organic acids quantitated by age groups in a healthy pediatric population. Clin Chem. 1977 Sep;23(9):1734–1738. [PubMed] [Google Scholar]
- VEST M. F., ROSSIER R. DETOXIFICATION IN THE NEWBORN: THE ABILITY OF THE NEWBORN INFANT TO FORM CONJUGATES WITH GLUCURONIC ACID, GLYCINE, ACETATE AND GLUTATHIONE. Ann N Y Acad Sci. 1963 Dec 30;111:183–198. doi: 10.1111/j.1749-6632.1963.tb36958.x. [DOI] [PubMed] [Google Scholar]
- Van Der Werf P., Stephani R. A., Meister A. Accumulation of 5-oxoproline in mouse tissues after inhibition of 5-oxoprolinase and administration of amino acids: evidence for function of the gamma-glutamyl cycle. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1026–1029. doi: 10.1073/pnas.71.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLF L. I., NORMAN A. P. The urinary excretion of amino acids and sugars in early infancy. J Pediatr. 1957 Mar;50(3):271–295. doi: 10.1016/s0022-3476(57)80025-0. [DOI] [PubMed] [Google Scholar]
- Worthington-White D. A., Behnke M., Gross S. Premature infants require additional folate and vitamin B-12 to reduce the severity of the anemia of prematurity. Am J Clin Nutr. 1994 Dec;60(6):930–935. doi: 10.1093/ajcn/60.6.930. [DOI] [PubMed] [Google Scholar]
- van der Werf P., Meister A. The metabolic formation and utilization of 5-oxo-L-proline (L-pyroglutamate, L-pyrrolidone carboxylate). Adv Enzymol Relat Areas Mol Biol. 1975;43:519–556. doi: 10.1002/9780470122884.ch7. [DOI] [PubMed] [Google Scholar]