Abstract
AIMS—To improve energy intake in sick very low birthweight (VLBW) infants; to decrease growth problems, lessen pulmonary morbidity, shorten hospital stay, and avoid possible feeding related morbidity. Morbidity in VLBW infants thought to be associated with parenteral and enteral feeding includes bronchopulmonary dysplasia, necrotising enterocolitis, septicaemia, cholestasis and osteopenia of prematurity. METHODS—A prospective randomised controlled trial (RCT) comparing two types of nutritional intervention was performed involving 125 sick VLBW infants in the setting of a regional neonatal intensive care unit. Babies were randomly allocated to either an aggressive nutritional regimen (group A) or a control group (group B). Babies in group B received a conservative nutritional regimen while group A received a package of more aggressive parenteral and enteral nutrition. Statistical analysis was done using Student's t test, the Mann-Whitney U test, the χ2 test and logistic regression. RESULTS—There was an excess of sicker babies in group A, as measured by initial disease severity (P <0.01), but mean total energy intakes were significantly higher (P <0.001) in group A at days 3 to 42 while receiving total or partial parenteral nutrition. Survival and the incidences of bronchopulmonary dysplasia, septicaemia, cholestasis, osteopenia and necrotising enterocolitis were similar in both groups. Growth in early life and at discharge from hospital was significantly better in babies in group A. There were no decreases in pulmonary morbidity or hospital stay. CONCLUSION—Nutritional intake in sick VLBW infants can be improved without increasing the risk of adverse clinical or metabolic sequelae. Improved nutritional intake resulted in better growth, both in the early neonatal period and at hospital discharge, but did not decrease pulmonary morbidity or shorten hospital stay. Keywords: very low birthweight infant; nutrition; bronchopulmonary dysplasia; necrotising enterocolitis
Full Text
The Full Text of this article is available as a PDF (161.2 KB).
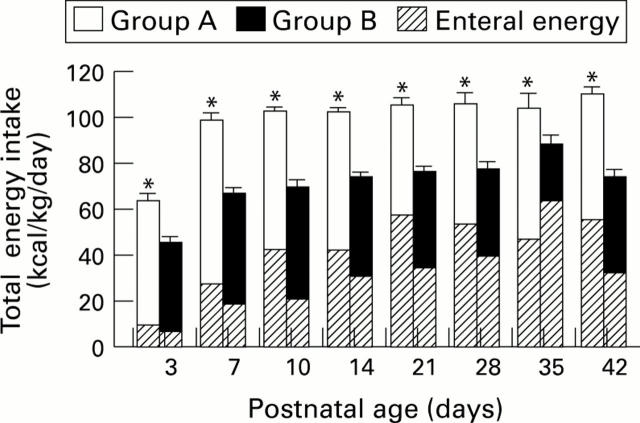
Figure 1 .
Mean (SEM) total enteral and parenteral energy intakes (kcal /kg/day) in groups A and B while receiving parenteral nutrition from postnatal ages of 3 to 42 days. * P < 0.001.
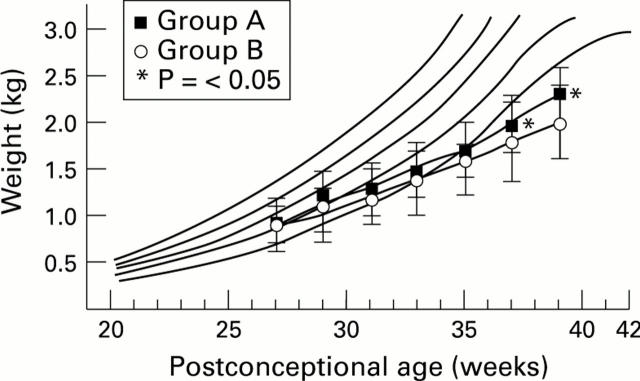
Figure 2 .
Mean (SD) weight (kg) in groups A and B from birth to postnatal age of 12 weeks, plotted on a Keen and Pearse weight chart for boys.45
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwaidh M. H., Bowden L., Shaw B., Ryan S. W. Randomised trial of effect of delayed intravenous lipid administration on chronic lung disease in preterm neonates. J Pediatr Gastroenterol Nutr. 1996 Apr;22(3):303–306. doi: 10.1097/00005176-199604000-00013. [DOI] [PubMed] [Google Scholar]
- Andrew G., Chan G., Schiff D. Lipid metabolism in the neonate. II. The effect of Intralipid on bilirubin binding in vitro and in vivo. J Pediatr. 1976 Feb;88(2):279–284. [PubMed] [Google Scholar]
- Barker D. J. Fetal growth and adult disease. Br J Obstet Gynaecol. 1992 Apr;99(4):275–276. doi: 10.1111/j.1471-0528.1992.tb13719.x. [DOI] [PubMed] [Google Scholar]
- Berseth C. L. Effect of early feeding on maturation of the preterm infant's small intestine. J Pediatr. 1992 Jun;120(6):947–953. doi: 10.1016/s0022-3476(05)81969-9. [DOI] [PubMed] [Google Scholar]
- Cairns P. A., Wilson D. C., McClure B. G., Halliday H. L., McReid M. Percutaneous central venous catheter use in the very low birth weight neonate. Eur J Pediatr. 1995 Feb;154(2):145–147. doi: 10.1007/BF01991919. [DOI] [PubMed] [Google Scholar]
- Collins J. W., Jr, Hoppe M., Brown K., Edidin D. V., Padbury J., Ogata E. S. A controlled trial of insulin infusion and parenteral nutrition in extremely low birth weight infants with glucose intolerance. J Pediatr. 1991 Jun;118(6):921–927. doi: 10.1016/s0022-3476(05)82212-7. [DOI] [PubMed] [Google Scholar]
- Cooke R. W. Factors associated with chronic lung disease in preterm infants. Arch Dis Child. 1991 Jul;66(7 Spec No):776–779. doi: 10.1136/adc.66.7_spec_no.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey A. M., Wagner C. L., Cox C., Kendig J. W. Feeding premature infants while low umbilical artery catheters are in place: a prospective, randomized trial. J Pediatr. 1994 May;124(5 Pt 1):795–799. doi: 10.1016/s0022-3476(05)81376-9. [DOI] [PubMed] [Google Scholar]
- Dunn L., Hulman S., Weiner J., Kliegman R. Beneficial effects of early hypocaloric enteral feeding on neonatal gastrointestinal function: preliminary report of a randomized trial. J Pediatr. 1988 Apr;112(4):622–629. doi: 10.1016/s0022-3476(88)80185-9. [DOI] [PubMed] [Google Scholar]
- Dweck H. S., Cassady G. Glucose intolerance in infants of very low birth weight. I. Incidence of hyperglycemia in infants of birth weights 1,100 grams or less. Pediatrics. 1974 Feb;53(2):189–195. [PubMed] [Google Scholar]
- Edelman N. H., Rucker R. B., Peavy H. H. NIH workshop summary: Nutrition and the respiratory system. Chronic obstructive pulmonary disease (COPD). Am Rev Respir Dis. 1986 Aug;134(2):347–352. doi: 10.1164/arrd.1986.134.2.347. [DOI] [PubMed] [Google Scholar]
- Fenton T. R., McMillan D. D., Sauve R. S. Nutrition and growth analysis of very low birth weight infants. Pediatrics. 1990 Sep;86(3):378–383. [PubMed] [Google Scholar]
- Frank L., Sosenko I. R. Undernutrition as a major contributing factor in the pathogenesis of bronchopulmonary dysplasia. Am Rev Respir Dis. 1988 Sep;138(3):725–729. doi: 10.1164/ajrccm/138.3.725. [DOI] [PubMed] [Google Scholar]
- Freeman J., Goldmann D. A., Smith N. E., Sidebottom D. G., Epstein M. F., Platt R. Association of intravenous lipid emulsion and coagulase-negative staphylococcal bacteremia in neonatal intensive care units. N Engl J Med. 1990 Aug 2;323(5):301–308. doi: 10.1056/NEJM199008023230504. [DOI] [PubMed] [Google Scholar]
- Gilbertson N., Kovar I. Z., Cox D. J., Crowe L., Palmer N. T. Introduction of intravenous lipid administration on the first day of life in the very low birth weight neonate. J Pediatr. 1991 Oct;119(4):615–623. doi: 10.1016/s0022-3476(05)82416-3. [DOI] [PubMed] [Google Scholar]
- Hammerman C., Aramburo M. J. Decreased lipid intake reduces morbidity in sick premature neonates. J Pediatr. 1988 Dec;113(6):1083–1088. doi: 10.1016/s0022-3476(88)80587-0. [DOI] [PubMed] [Google Scholar]
- Helbock H. J., Motchnik P. A., Ames B. N. Toxic hydroperoxides in intravenous lipid emulsions used in preterm infants. Pediatrics. 1993 Jan;91(1):83–87. [PubMed] [Google Scholar]
- Kashyap S., Schulze K. F., Forsyth M., Zucker C., Dell R. B., Ramakrishnan R., Heird W. C. Growth, nutrient retention, and metabolic response in low birth weight infants fed varying intakes of protein and energy. J Pediatr. 1988 Oct;113(4):713–721. doi: 10.1016/s0022-3476(88)80388-3. [DOI] [PubMed] [Google Scholar]
- Keen D. V., Pearse R. G. Weight, length, and head circumference curves for boys and girls of between 20 and 42 weeks' gestation. Arch Dis Child. 1988 Oct;63(10 Spec No):1170–1172. doi: 10.1136/adc.63.10_spec_no.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine G. M., Deren J. J., Steiger E., Zinno R. Role of oral intake in maintenance of gut mass and disaccharide activity. Gastroenterology. 1974 Nov;67(5):975–982. [PubMed] [Google Scholar]
- Lima L. A., Murphy J. F., Stansbie D., Rowlandson P., Gray O. P. Neonatal parenteral nutrition with a fat emulsion containing medium chain triglycerides. Acta Paediatr Scand. 1988 May;77(3):332–339. doi: 10.1111/j.1651-2227.1988.tb10657.x. [DOI] [PubMed] [Google Scholar]
- Lucas A., Bloom S. R., Aynsley-Green A. Gut hormones and 'minimal enteral feeding'. Acta Paediatr Scand. 1986 Sep;75(5):719–723. doi: 10.1111/j.1651-2227.1986.tb10280.x. [DOI] [PubMed] [Google Scholar]
- Lucas A., Morley R., Cole T. J., Gore S. M., Lucas P. J., Crowle P., Pearse R., Boon A. J., Powell R. Early diet in preterm babies and developmental status at 18 months. Lancet. 1990 Jun 23;335(8704):1477–1481. doi: 10.1016/0140-6736(90)93026-l. [DOI] [PubMed] [Google Scholar]
- Meetze W. H., Valentine C., McGuigan J. E., Conlon M., Sacks N., Neu J. Gastrointestinal priming prior to full enteral nutrition in very low birth weight infants. J Pediatr Gastroenterol Nutr. 1992 Aug;15(2):163–170. doi: 10.1097/00005176-199208000-00011. [DOI] [PubMed] [Google Scholar]
- Newell S. J., Morgan M. E., Durbin G. M., Booth I. W., McNeish A. S. Does mechanical ventilation precipitate gastro-oesophageal reflux during enteral feeding? Arch Dis Child. 1989 Oct;64(10 Spec No):1352–1355. doi: 10.1136/adc.64.10_spec_no.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag S. G., LaGamma E. F., Reisen C. E., Ferrentino F. L. Early enteral feeding does not affect the incidence of necrotizing enterocolitis. Pediatrics. 1986 Mar;77(3):275–280. [PubMed] [Google Scholar]
- Periera G. R., Fox W. W., Stanley C. A., Baker L., Schwartz J. G. Decreased oxygenation and hyperlipemia during intravenous fat infusions in premature infants. Pediatrics. 1980 Jul;66(1):26–30. [PubMed] [Google Scholar]
- Schmidt-Sommerfeld E., Penn D., Wolf H. Carnitine blood concentrations and fat utilization in parenterally alimented premature newborn infants. J Pediatr. 1982 Feb;100(2):260–264. doi: 10.1016/s0022-3476(82)80652-5. [DOI] [PubMed] [Google Scholar]
- Sittlington N., Tubman R., Halliday H. L. Surfactant replacement therapy for severe neonatal respiratory distress syndrome: implications for nursing care. Midwifery. 1991 Mar;7(1):20–24. doi: 10.1016/s0266-6138(05)80130-5. [DOI] [PubMed] [Google Scholar]
- Slagle T. A., Gross S. J. Effect of early low-volume enteral substrate on subsequent feeding tolerance in very low birth weight infants. J Pediatr. 1988 Sep;113(3):526–531. doi: 10.1016/s0022-3476(88)80646-2. [DOI] [PubMed] [Google Scholar]
- Sosenko I. R., Rodriguez-Pierce M., Bancalari E. Effect of early initiation of intravenous lipid administration on the incidence and severity of chronic lung disease in premature infants. J Pediatr. 1993 Dec;123(6):975–982. doi: 10.1016/s0022-3476(05)80397-x. [DOI] [PubMed] [Google Scholar]
- Stenmark K. R., Eyzaguirre M., Westcott J. Y., Henson P. M., Murphy R. C. Potential role of eicosanoids and PAF in the pathophysiology of bronchopulmonary dysplasia. Am Rev Respir Dis. 1987 Sep;136(3):770–772. doi: 10.1164/ajrccm/136.3.770. [DOI] [PubMed] [Google Scholar]
- Tammela O. K., Koivisto M. E. Fluid restriction for preventing bronchopulmonary dysplasia? Reduced fluid intake during the first weeks of life improves the outcome of low-birth-weight infants. Acta Paediatr. 1992 Mar;81(3):207–212. doi: 10.1111/j.1651-2227.1992.tb12205.x. [DOI] [PubMed] [Google Scholar]
- Troche B., Harvey-Wilkes K., Engle W. D., Nielsen H. C., Frantz I. D., 3rd, Mitchell M. L., Hermos R. J. Early minimal feedings promote growth in critically ill premature infants. Biol Neonate. 1995;67(3):172–181. doi: 10.1159/000244160. [DOI] [PubMed] [Google Scholar]
- Wilson D. C., McClure G. Energy requirements in sick preterm babies. Acta Paediatr Suppl. 1994 Dec;405:60–64. doi: 10.1111/j.1651-2227.1994.tb13400.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. C., McClure G., Halliday H. L., Reid M. M., Dodge J. A. Nutrition and bronchopulmonary dysplasia. Arch Dis Child. 1991 Jan;66(1 Spec No):37–38. doi: 10.1136/adc.66.1_spec_no.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. C. Nutrition of the preterm baby. Br J Obstet Gynaecol. 1995 Nov;102(11):854–860. doi: 10.1111/j.1471-0528.1995.tb10871.x. [DOI] [PubMed] [Google Scholar]
- Yunis K. A., Oh W. Effects of intravenous glucose loading on oxygen consumption, carbon dioxide production, and resting energy expenditure in infants with bronchopulmonary dysplasia. J Pediatr. 1989 Jul;115(1):127–132. doi: 10.1016/s0022-3476(89)80347-6. [DOI] [PubMed] [Google Scholar]