Abstract
AIMS—To determine if the failure of neonatal pulmonary arteries to dilate is due to a lack of nitric oxide synthase (NOS). METHODS—A monoclonal antibody to endothelial NOS was used to demonstrate the distribution and density of NOS in the developing porcine lung after a period in hypobaric hypoxia. Newborn piglets were made hypertensive by exposure to hypobaric hypoxia (50.8 kPa) from < 5 minutes of age to 2.5 days of age, 3-6 days of age or 14-17 days of age. A semiquantitative scoring system was used to assess the distribution of endothelial NOS by light microscopy. RESULTS—NOS was present in the arteries in all hypoxic animals. However, hypoxia from birth caused a reduction in NOS compared with those lungs normal at birth and those normal at 3 days. Hypoxia from 3-6 days led to a high density of NOS compared with normal lungs at 6 days. Hypoxia from 14-17 days had little effect on the amount of NOS. On recovery in room air after exposure to hypoxia from birth there was a transient increase in endothelial NOS after three days of recovery, mirroring that seen at three days in normal animals. CONCLUSIONS—Suppression of NOS production in the first few days of life may contribute to pulmonary hypertension in neonates. Keywords: pulmonary circulation; nitric oxide synthase; hypoxia; endothelium; piglets
Full Text
The Full Text of this article is available as a PDF (138.3 KB).
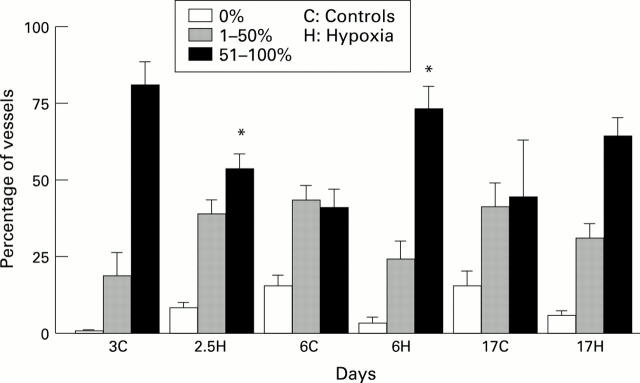
Figure 1 .
Mean (SD) percentage of arteries at bronchiolar level containing no endothelial NOS, containing up to 50%, or 51-100% in hypoxic and control piglets. *P<0.05 compared with controls of the same age.
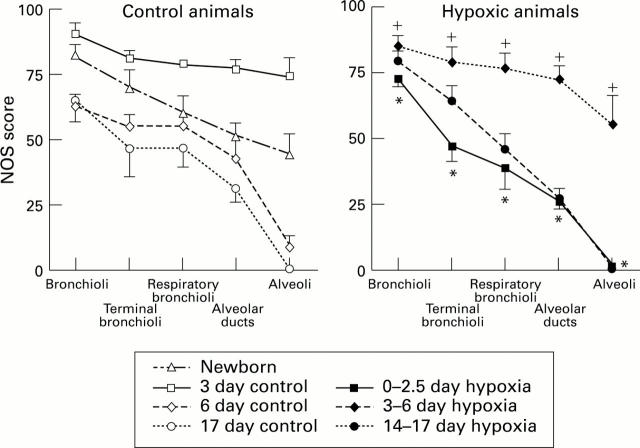
Figure 2 .
NOS score (SD) for control and hypoxic animals in arteries at the level of the bronchioli, terminal bronchi, respiratory bronchioli, alveolar ducts, and alveoli. *P<0.05 compared with control 3 day olds; +P<0.05 compared with control 6 day olds.
Figure 3 .
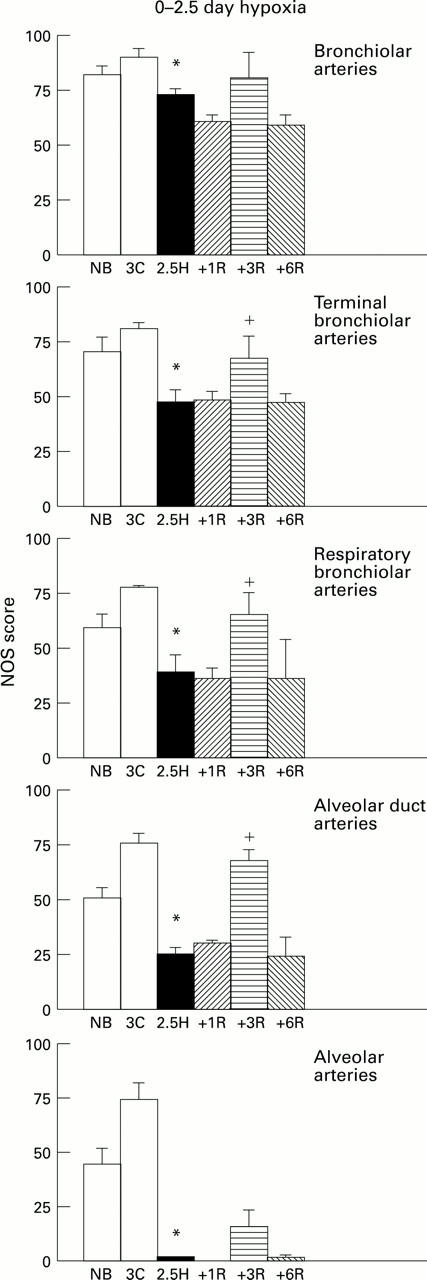
NOS score (SD) for control (C), hypoxic (H), and recovery (R) animals at each arterial level in 0-2.5 day hypoxic group. *P<0.05 compared with control 3 day olds; +P<0.05 compared with 2.5 day hypoxic animals.
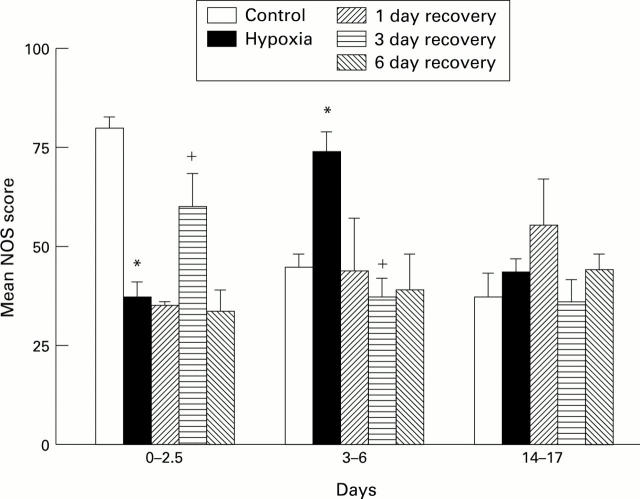
Figure 4 .
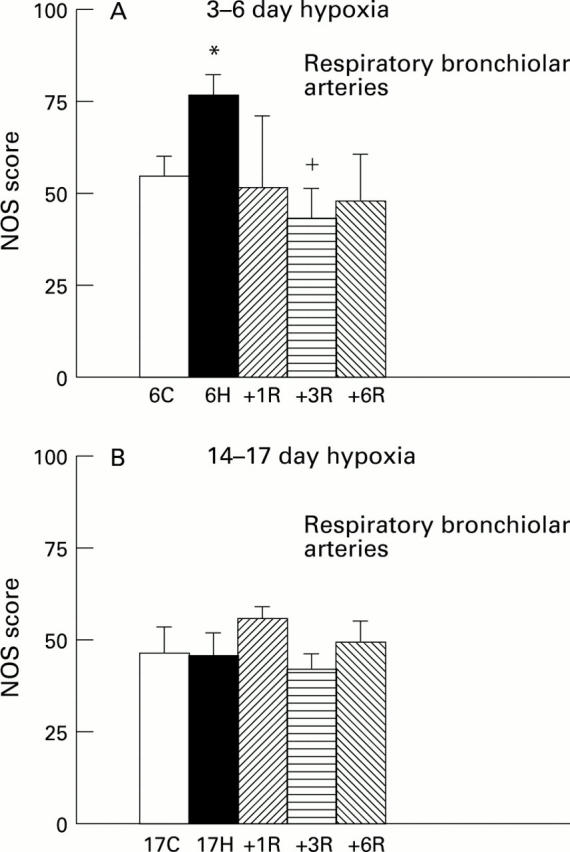
NOS score for control (C), hypoxic (H), and recovery (R) animals at respiratory bronchiolar level in (A) 3-6 day hypoxic group and in (B) 14-17 day hypoxic group. *P<0.05 compared with 6 day old controls; +P<0.05 compared with 6 day hypoxia.
Figure 5 .
Mean (SD) NOS score for the three hypoxic groups. *P<0.05 compared with controls of the same age; +P<0.05 compared with hypoxia.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adnot S., Raffestin B., Eddahibi S., Braquet P., Chabrier P. E. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J Clin Invest. 1991 Jan;87(1):155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K. M., Haworth S. G. Impaired adaptation of pulmonary circulation to extrauterine life in newborn pigs exposed to hypoxia: an ultrastructural study. J Pathol. 1986 Nov;150(3):205–212. doi: 10.1002/path.1711500309. [DOI] [PubMed] [Google Scholar]
- Bansal V., Toga H., Raj J. U. Tone dependent nitric oxide production in ovine vessels in vitro. Respir Physiol. 1993 Aug;93(2):249–260. doi: 10.1016/0034-5687(93)90009-y. [DOI] [PubMed] [Google Scholar]
- Etches P. C., Finer N. N., Barrington K. J., Graham A. J., Chan W. K. Nitric oxide reverses acute hypoxic pulmonary hypertension in the newborn piglet. Pediatr Res. 1994 Jan;35(1):15–19. doi: 10.1203/00006450-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Fukaya Y., Ohhashi T. Acetylcholine- and flow-induced production and release of nitric oxide in arterial and venous endothelial cells. Am J Physiol. 1996 Jan;270(1 Pt 2):H99–106. doi: 10.1152/ajpheart.1996.270.1.H99. [DOI] [PubMed] [Google Scholar]
- Gao Y., Zhou H., Raj J. U. Heterogeneity in role of endothelium-derived NO in pulmonary arteries and veins of full-term fetal lambs. Am J Physiol. 1995 Apr;268(4 Pt 2):H1586–H1592. doi: 10.1152/ajpheart.1995.268.4.H1586. [DOI] [PubMed] [Google Scholar]
- Hall S. M., Haworth S. G. Normal adaptation of pulmonary arterial intima to extrauterine life in the pig: ultrastructural studies. J Pathol. 1986 May;149(1):55–66. doi: 10.1002/path.1711490111. [DOI] [PubMed] [Google Scholar]
- Haworth S. G., Hislop A. A. Effect of hypoxia on adaptation of the pulmonary circulation to extra-uterine life in the pig. Cardiovasc Res. 1982 Jun;16(6):293–303. doi: 10.1093/cvr/16.6.293. [DOI] [PubMed] [Google Scholar]
- Heath D., Smith P., Rios Dalenz J., Williams D., Harris P. Small pulmonary arteries in some natives of La Paz, Bolivia. Thorax. 1981 Aug;36(8):599–604. doi: 10.1136/thx.36.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop A. A., Haworth S. G. Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol. 1990;9(3):152–161. doi: 10.1002/ppul.1950090306. [DOI] [PubMed] [Google Scholar]
- Hislop A. A., Springall D. R., Buttery L. D., Pollock J. S., Haworth S. G. Abundance of endothelial nitric oxide synthase in newborn intrapulmonary arteries. Arch Dis Child Fetal Neonatal Ed. 1995 Jul;73(1):F17–F21. doi: 10.1136/fn.73.1.f17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson T. C., Hampl V., Weir E. K., Nelson D. P., Archer S. L. Increased endothelium-derived NO in hypertensive pulmonary circulation of chronically hypoxic rats. J Appl Physiol (1985) 1994 Feb;76(2):933–940. doi: 10.1152/jappl.1994.76.2.933. [DOI] [PubMed] [Google Scholar]
- Kinsella J. P., Neish S. R., Shaffer E., Abman S. H. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992 Oct 3;340(8823):819–820. doi: 10.1016/0140-6736(92)92687-b. [DOI] [PubMed] [Google Scholar]
- McQueston J. A., Kinsella J. P., Ivy D. D., McMurtry I. F., Abman S. H. Chronic pulmonary hypertension in utero impairs endothelium-dependent vasodilation. Am J Physiol. 1995 Jan;268(1 Pt 2):H288–H294. doi: 10.1152/ajpheart.1995.268.1.H288. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Endothelial and subintimal changes in rat hilar pulmonary artery during recovery from hypoxia. A quantitative ultrastructural study. Lab Invest. 1980 Jun;42(6):603–615. [PubMed] [Google Scholar]
- Ohno M., Gibbons G. H., Dzau V. J., Cooke J. P. Shear stress elevates endothelial cGMP. Role of a potassium channel and G protein coupling. Circulation. 1993 Jul;88(1):193–197. doi: 10.1161/01.cir.88.1.193. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. D., Polaner D. M., Lang P., Zapol W. M. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992 Oct 3;340(8823):818–819. doi: 10.1016/0140-6736(92)92686-a. [DOI] [PubMed] [Google Scholar]
- Russ R. D., Walker B. R. Maintained endothelium-dependent pulmonary vasodilation following chronic hypoxia in the rat. J Appl Physiol (1985) 1993 Jan;74(1):339–344. doi: 10.1152/jappl.1993.74.1.339. [DOI] [PubMed] [Google Scholar]
- Shaul P. W., Wells L. B., Horning K. M. Acute and prolonged hypoxia attenuate endothelial nitric oxide production in rat pulmonary arteries by different mechanisms. J Cardiovasc Pharmacol. 1993 Dec;22(6):819–827. doi: 10.1097/00005344-199312000-00007. [DOI] [PubMed] [Google Scholar]
- Shu S. Y., Ju G., Fan L. Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988 Feb 29;85(2):169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Steinhorn R. H., Russell J. A., Morin F. C., 3rd Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol. 1995 Apr;268(4 Pt 2):H1483–H1489. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- Wanstall J. C., Hughes I. E., O'Donnell S. R. Evidence that nitric oxide from the endothelium attenuates inherent tone in isolated pulmonary arteries from rats with hypoxic pulmonary hypertension. Br J Pharmacol. 1995 Jan;114(1):109–114. doi: 10.1111/j.1476-5381.1995.tb14913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]