Abstract
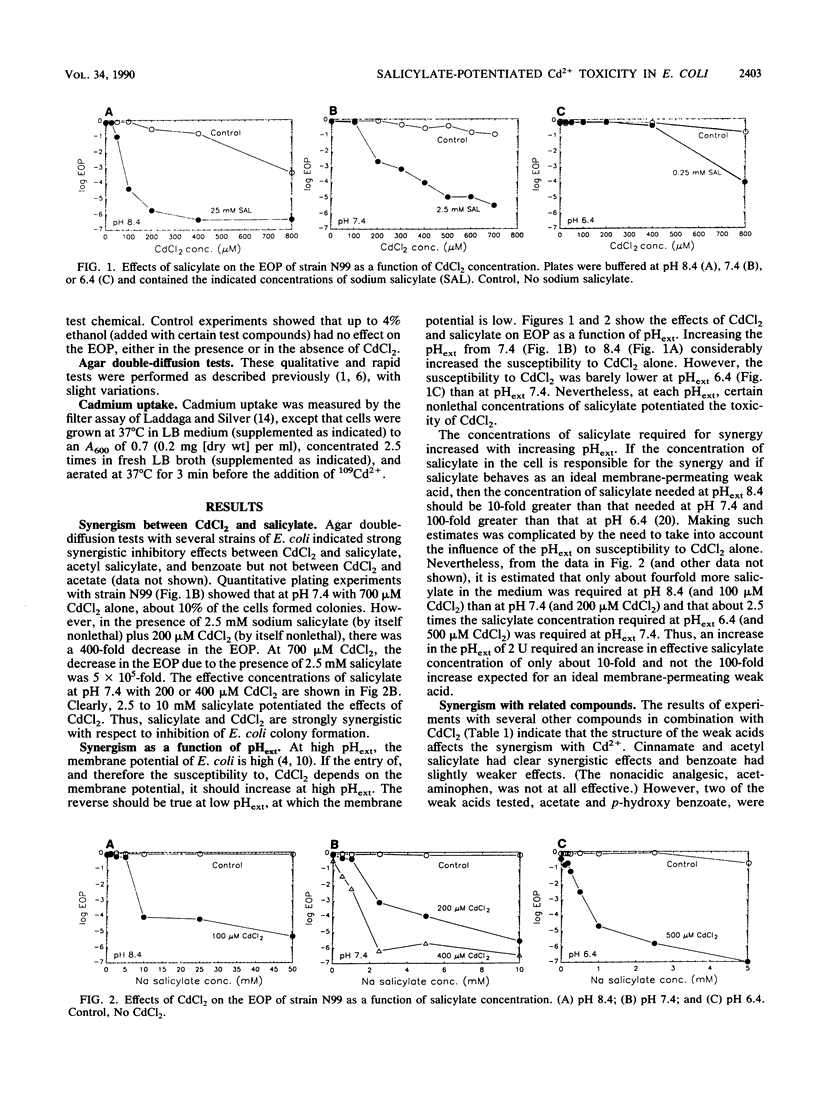
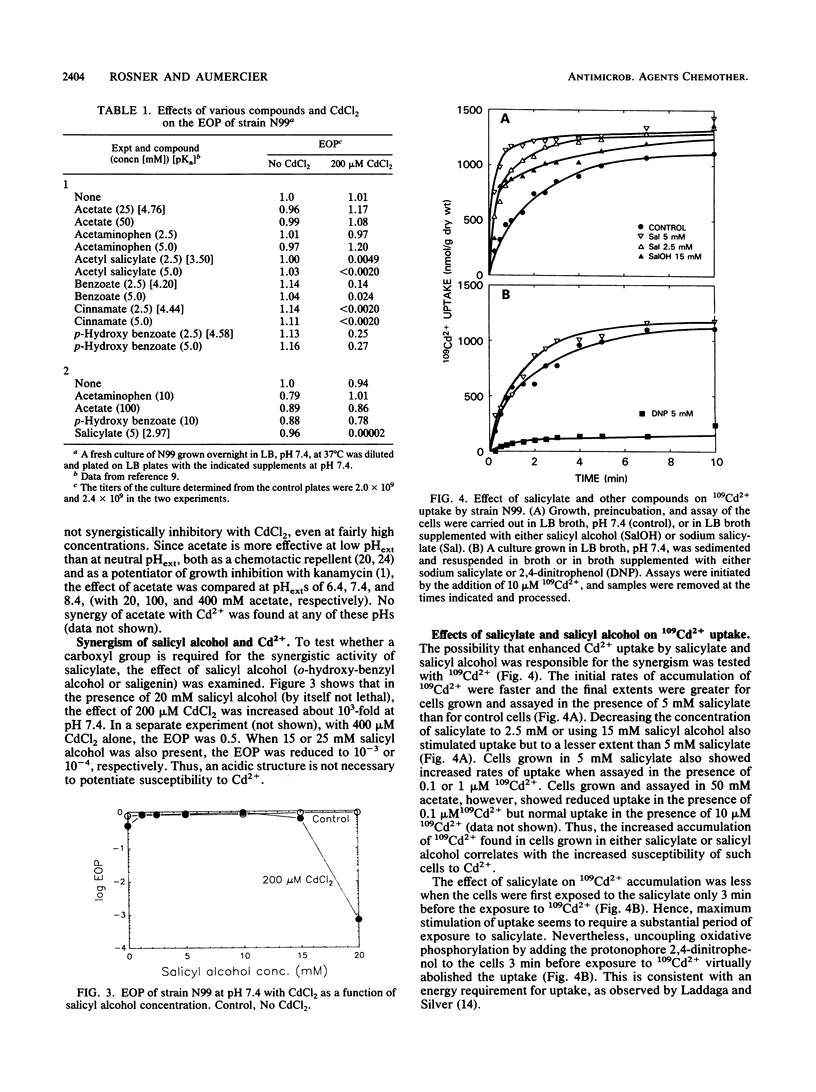
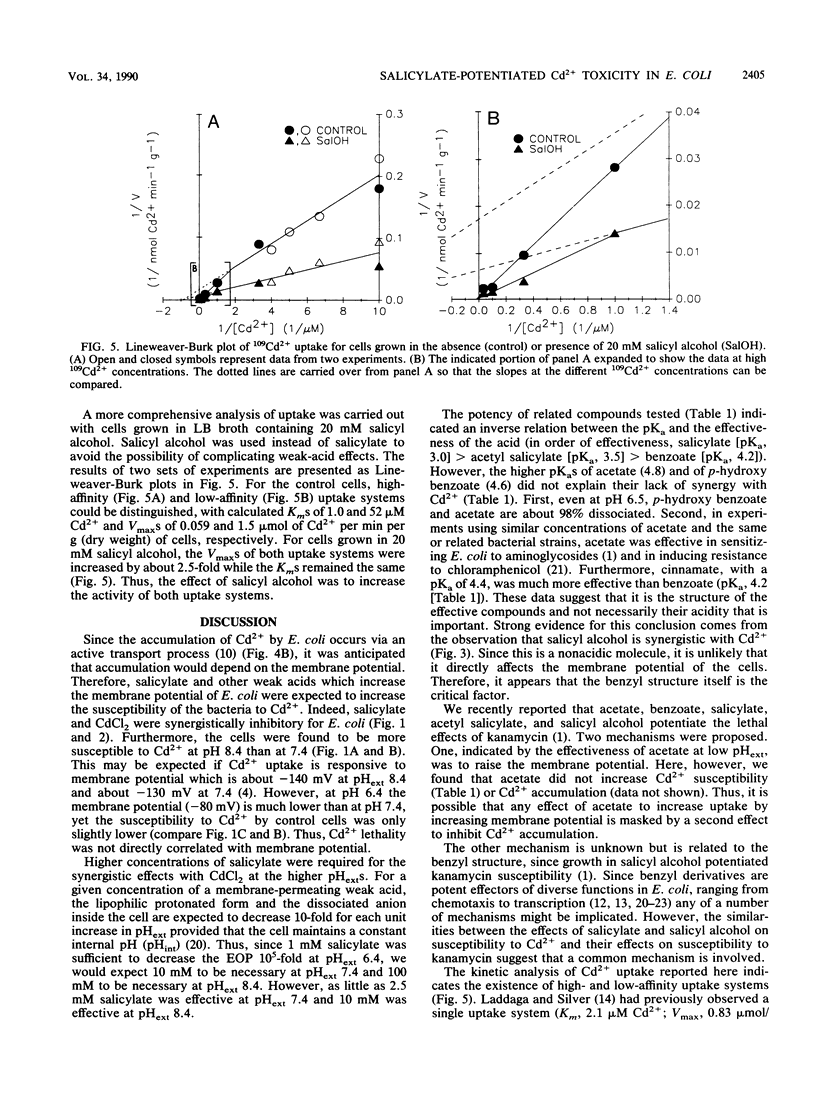
The toxicity of Cd2+ in Escherichia coli K-12 was potentiated by salicylate and several related compounds. The efficiency of plating on Luria broth plates was reduced by more than 10(5)-fold when 10 mM salicylate and 200 microM CdCl2 were present simultaneously but was unaffected when either compound was present by itself. Synergistic effects were found at pH 7.4 with certain other weak acids (acetyl salicylate [aspirin], benzoate, and cinnamate) and with a nonacidic salicylate analog, salicyl alcohol, but not with acetate or p-hydroxy benzoate. Thus, the synergism with Cd2+ is determined by the structure of the compounds and not merely by their acidity. The kinetics of 109Cd2+ uptake by cells grown and assayed in broth indicated the presence of two uptake systems with Kms of 1 and 52 microM Cd2+ and Vmaxs of 0.059 and 1.5 mumol of Cd2+ per min per g of cells, respectively. The kinetics of uptake for cells grown and assayed with 20 mM salicyl alcohol showed 2.5-fold increases in the Vmaxs of both systems but no change in the Kms. Salicylate-grown cells also exhibited increased rates of 109Cd2+ uptake by both systems. Thus, enhanced uptake of Cd2+ may be responsible for the potentiation of Cd2+ toxicity by salicylate and salicyl alcohol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aumercier M., Murray D. M., Rosner J. L. Potentiation of susceptibility to aminoglycosides by salicylate in Escherichia coli. Antimicrob Agents Chemother. 1990 May;34(5):786–791. doi: 10.1128/aac.34.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damper P. D., Epstein W. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob Agents Chemother. 1981 Dec;20(6):803–808. doi: 10.1128/aac.20.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch D. C., Salvacion F. F., Epstein W. Tetracycline resistance element of pBR322 mediates potassium transport. J Bacteriol. 1984 Dec;160(3):1188–1190. doi: 10.1128/jb.160.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Murray D. M., Chai T., Rosner J. L. Decreased permeation of cephalosporins through the outer membrane of Escherichia coli grown in salicylates. Antimicrob Agents Chemother. 1989 Apr;33(4):412–417. doi: 10.1128/aac.33.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. K., Buckingham J. M., Hanners J. L., Hildebrand C. E., Walters R. A. Plasmid-conferred tetracycline resistance confers collateral cadmium sensitivity of E. coli cells. Plasmid. 1982 Jul;8(1):86–88. doi: 10.1016/0147-619x(82)90044-0. [DOI] [PubMed] [Google Scholar]
- Griffith J. K., Kogoma T., Corvo D. L., Anderson W. L., Kazim A. L. An N-terminal domain of the tetracycline resistance protein increases susceptibility to aminoglycosides and complements potassium uptake defects in Escherichia coli. J Bacteriol. 1988 Feb;170(2):598–604. doi: 10.1128/jb.170.2.598-604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Stoichiometry of the H+-ATPase of growing and resting, aerobic Escherichia coli. Biochemistry. 1982 Oct 26;21(22):5534–5538. doi: 10.1021/bi00265a024. [DOI] [PubMed] [Google Scholar]
- Khazaeli M. B., Mitra R. S. Cadmium-binding component in Escherichia coli during accommodation to low levels of this ion. Appl Environ Microbiol. 1981 Jan;41(1):46–50. doi: 10.1128/aem.41.1.46-50.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. L., West R. W., Jr, Ink B. S., Kline P. M., Rodriguez R. L. Benzyl derivative facilitation of transcription in Escherichia coli at the ara and lac operon promoters: metabolite gene regulation (MGR). Mol Gen Genet. 1984;193(2):340–348. doi: 10.1007/BF00330691. [DOI] [PubMed] [Google Scholar]
- Laddaga R. A., Silver S. Cadmium uptake in Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1100–1105. doi: 10.1128/jb.162.3.1100-1105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin T. L., Corvo D. L., Gill J. H., Griffith J. K. Enhanced gentamicin killing of Escherichia coli by tet gene expression. Antimicrob Agents Chemother. 1989 Feb;33(2):230–232. doi: 10.1128/aac.33.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin T. L., Davis G. E., Anderson W. L., Moyzis R. K., Griffith J. K. Aminoglycoside uptake increased by tet gene expression. Antimicrob Agents Chemother. 1989 Sep;33(9):1549–1552. doi: 10.1128/aac.33.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitra R. S., Bernstein I. A. Single-strand breakage in DNA of Escherichia coli exposed to Cd2+. J Bacteriol. 1978 Jan;133(1):75–80. doi: 10.1128/jb.133.1.75-80.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. S., Gray R. H., Chin B., Bernstein I. A. Molecular mechanisms of accommodation in Escherichia coli to toxic levels of Cd2+. J Bacteriol. 1975 Mar;121(3):1180–1188. doi: 10.1128/jb.121.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske D. R., Adler J. Change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellents. J Bacteriol. 1981 Mar;145(3):1196–1208. doi: 10.1128/jb.145.3.1196-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Nonheritable resistance to chloramphenicol and other antibiotics induced by salicylates and other chemotactic repellents in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8771–8774. doi: 10.1073/pnas.82.24.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Gonzalez T. N., Bartholomew F. M., Holt N. J. Mu d-directed lacZ fusions regulated by low pH in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3001–3006. doi: 10.1128/jb.169.7.3001-3006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski J. L., Macnab R. M., Alger J. R., Castle A. M. Effects of pH and repellent tactic stimuli on protein methylation levels in Escherichia coli. J Bacteriol. 1982 Oct;152(1):384–399. doi: 10.1128/jb.152.1.384-399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Stock J. B., Koshland D. E., Jr Role of membrane potential and calcium in chemotactic sensing by bacteria. J Mol Biol. 1981 Jun 25;149(2):241–257. doi: 10.1016/0022-2836(81)90300-4. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


