Abstract
AIM—To investigate if early changes in concentrations of proinflammatory cytokines in tracheobronchial aspirate fluid (TAF) from preterm infants could be used to detect infants at risk of chronic lung disease (CLD) and help in the selection of patients for early steroid treatment. METHODS—Twenty eight preterm infants less than 34 weeks of gestation (median 26 weeks) were intubated and daily measurements of TAF concentrations of tumour necrosis factor α (TNFα) and the interleukins IL-1β, IL-6, and IL-8 were made, using enzyme immunoassay techniques. RESULTS—Seventeen of the infants developed CLD. The infants who developed CLD had significantly increased concentrations of TNFα, IL-1ß, IL-6 on days 2 and 3. TNFα, IL-6, and IL-8 concentrations were significantly related to gestational age and duration of supplemental oxygen; TNFα, IL-6, and IL-8 concentrations also correlated with length of time on the ventilator. CONCLUSION—These data indicate that tracheobronchial aspirate fluid cytokine concentrations may be used as a predictor of subsequent CLD and may help select a group of preterm infants at high risk of developing CLD for early treatment. Keywords: cytokines; chronic lung disease; tracheobronchial aspirate fluid; mechanical ventilation
Full Text
The Full Text of this article is available as a PDF (140.0 KB).
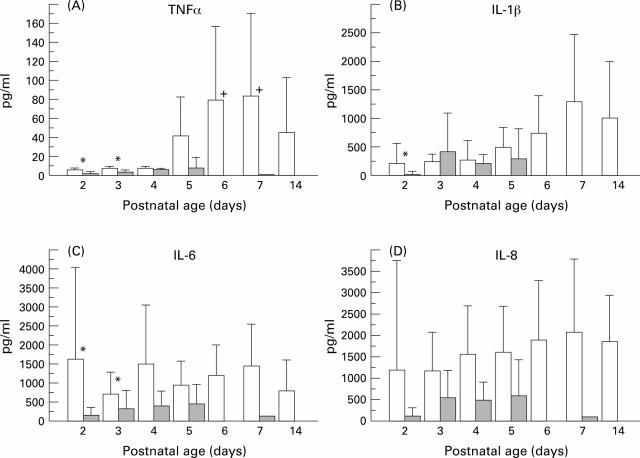
Figure 1 .
TAF fluid concentrations of proinflammatory cytokines in infants who subsequently developed CLD and infants with uncomplicated RDS (shaded area). Bars indicate mean values with 95% confidence intervals, on separate days. (A) TNFα concentrations: *denotes p<0.05 on days 2 and 3 between CLD and RDS infants; + p>0.05 on days 6 and 7 compared with days 2 and 3 for CLD infants. (B) IL-1β antigen titres:*p <0.05 between RDS and CLD infants on day 2. (C) IL 6 concentrations: *p<0.05 on days 2 and 3 between the two groups of infants. (D) IL 8 concentrations
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon S., Grigg J., Silverman M. Pulmonary inflammatory cells in ventilated preterm infants: effect of surfactant treatment. Arch Dis Child. 1993 Jul;69(1 Spec No):44–48. doi: 10.1136/adc.69.1_spec_no.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi A., Viscardi R. M., Taciak V., Ensor J. E., McCrea K. A., Hasday J. D. Increased activity of interleukin-6 but not tumor necrosis factor-alpha in lung lavage of premature infants is associated with the development of bronchopulmonary dysplasia. Pediatr Res. 1994 Aug;36(2):244–252. doi: 10.1203/00006450-199408000-00017. [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Sosenko I. R., Chandar J., Hummler H., Claure N., Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. 1996 Apr;128(4):470–478. doi: 10.1016/s0022-3476(96)70356-6. [DOI] [PubMed] [Google Scholar]
- Grigg J. M., Barber A., Silverman M. Increased levels of bronchoalveolar lavage fluid interleukin-6 in preterm ventilated infants after prolonged rupture of membranes. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):782–786. doi: 10.1164/ajrccm/145.4_Pt_1.782. [DOI] [PubMed] [Google Scholar]
- Groneck P., Goetze-Speer B., Speer C. P. Inflammatory bronchopulmonary response of preterm infants with microbial colonisation of the airways at birth. Arch Dis Child Fetal Neonatal Ed. 1996 Jan;74(1):F51–F55. doi: 10.1136/fn.74.1.f51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneck P., Götze-Speer B., Oppermann M., Eiffert H., Speer C. P. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics. 1994 May;93(5):712–718. [PubMed] [Google Scholar]
- Groneck P., Speer C. P. Inflammatory mediators and bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. 1995 Jul;73(1):F1–F3. doi: 10.1136/fn.73.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneck P., Speer C. P. Interleukin-8 in pulmonary effluent fluid of preterm infants. J Pediatr. 1993 Nov;123(5):839–840. doi: 10.1016/s0022-3476(05)80884-4. [DOI] [PubMed] [Google Scholar]
- Hjalmarson O. Epidemiology and classification of acute, neonatal respiratory disorders. A prospective study. Acta Paediatr Scand. 1981 Nov;70(6):773–783. doi: 10.1111/j.1651-2227.1981.tb06228.x. [DOI] [PubMed] [Google Scholar]
- Jones C. A., Cayabyab R. G., Kwong K. Y., Stotts C., Wong B., Hamdan H., Minoo P., deLemos R. A. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996 Jun;39(6):966–975. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. Cytokines of the lung. Am Rev Respir Dis. 1990 Mar;141(3):765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- McColm J. R., McIntosh N. Interleukin-8 in bronchoalveolar lavage samples as predictor of chronic lung disease in premature infants. Lancet. 1994 Mar 19;343(8899):729–729. doi: 10.1016/s0140-6736(94)91606-3. [DOI] [PubMed] [Google Scholar]
- Merritt T. A., Cochrane C. G., Holcomb K., Bohl B., Hallman M., Strayer D., Edwards D. K., 3rd, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983 Aug;72(2):656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt T. A. Oxygen exposure in the newborn guinea pig lung lavage cell populations, chemotactic and elastase response: a possible relationship to neonatal bronchopulmonary dysplasia. Pediatr Res. 1982 Sep;16(9):798–805. doi: 10.1203/00006450-198209000-00018. [DOI] [PubMed] [Google Scholar]
- Murch S. H., MacDonald T. T., Wood C. B., Costeloe K. L. Tumour necrosis factor in the bronchoalveolar secretions of infants with the respiratory distress syndrome and the effect of dexamethasone treatment. Thorax. 1992 Jan;47(1):44–47. doi: 10.1136/thx.47.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northway W. H., Jr An introduction to bronchopulmonary dysplasia. Clin Perinatol. 1992 Sep;19(3):489–495. [PubMed] [Google Scholar]
- Rozycki H. J. Bronchoalveolar interleukin-1 beta in infants on day 1 of life. South Med J. 1994 Oct;87(10):991–996. doi: 10.1097/00007611-199410000-00005. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Campbell L. A., Merritt T. A., Long W. A., Gunkel J. H., Curstedt T., Robertson B. Effect of different surfactants on pulmonary group B streptococcal infection in premature rabbits. J Pediatr. 1994 Dec;125(6 Pt 1):939–947. doi: 10.1016/s0022-3476(05)82013-x. [DOI] [PubMed] [Google Scholar]
- Silverman M. Chronic lung disease of prematurity: are we too cautious with steroids? Eur J Pediatr. 1994;153(9 Suppl 2):S30–S35. doi: 10.1007/BF02179671. [DOI] [PubMed] [Google Scholar]
- Speer C. P., Götze B., Curstedt T., Robertson B. Phagocytic functions and tumor necrosis factor secretion of human monocytes exposed to natural porcine surfactant (Curosurf). Pediatr Res. 1991 Jul;30(1):69–74. doi: 10.1203/00006450-199107000-00015. [DOI] [PubMed] [Google Scholar]
- Tullus K., Noack G. W., Burman L. G., Nilsson R., Wretlind B., Brauner A. Elevated cytokine levels in tracheobronchial aspirate fluids from ventilator treated neonates with bronchopulmonary dysplasia. Eur J Pediatr. 1996 Feb;155(2):112–116. doi: 10.1007/BF02075762. [DOI] [PubMed] [Google Scholar]
- Watterberg K. L., Demers L. M., Scott S. M., Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996 Feb;97(2):210–215. [PubMed] [Google Scholar]
- Watts C. L., Fanaroff A. A., Bruce M. C. Elevation of fibronectin levels in lung secretions of infants with respiratory distress syndrome and development of bronchopulmonary dysplasia. J Pediatr. 1992 Apr;120(4 Pt 1):614–620. doi: 10.1016/s0022-3476(05)82492-8. [DOI] [PubMed] [Google Scholar]
- deLemos R. A., Coalson J. J. The contribution of experimental models to our understanding of the pathogenesis and treatment of bronchopulmonary dysplasia. Clin Perinatol. 1992 Sep;19(3):521–539. [PubMed] [Google Scholar]