Abstract
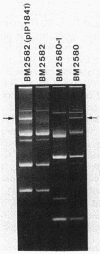
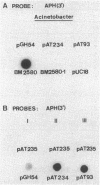
Acinetobacter baumannii BM2580 resistant to kanamycin and structurally related antibiotics, including amikacin, was isolated from a clinical specimen. A phosphocellulose paper-binding assay and DNA annealing studies indicated that resistance to aminoglycosides in BM2580 was due to synthesis of a new type of 3'-aminoglycoside phosphotransferase. The gene conferring resistance to kanamycin-amikacin in this strain was carried by a 63-kilobase plasmid, pIP1841, self-transferable to A. baumannii, A. haemolyticus, and A. lwoffii but not to Escherichia coli. The aminoglycoside resistance gene of pIP1841 was cloned in E. coli, where it was expressed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaerts G. P., Kaptijn G. M. Aminoglycoside phosphotransferase-II-mediated amikacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1981 Sep;20(3):344–350. doi: 10.1128/aac.20.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y. Une technique nouvelle d'étude de l'action bactéricide des associations d'antibiotiques: le transfert sur cellophane. Ann Inst Pasteur (Paris) 1957 Sep;93(3):289–299. [PubMed] [Google Scholar]
- Chopade B. A., Wise P. J., Towner K. J. Plasmid transfer and behaviour in Acinetobacter calcoaceticus EBF65/65. J Gen Microbiol. 1985 Oct;131(10):2805–2811. doi: 10.1099/00221287-131-10-2805. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Fiandt M. Aminoglycoside-modifying enzymes of Staphylococcus aureus; expression in Escherichia coli. Gene. 1980 May;9(3-4):247–269. doi: 10.1016/0378-1119(90)90326-m. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Weisblum B., Davies J. Aminoglycoside-modifying enzyme of an antibiotic-producing bacterium acts as a determinant of antibiotic resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):999–1003. doi: 10.1073/pnas.74.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud M., Kayser F. H., Bächi B. Transposon-mediated multiple antibiotic resistance in Acinetobacter strains. Antimicrob Agents Chemother. 1982 Aug;22(2):323–329. doi: 10.1128/aac.22.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowding J. E. A novel aminoglycoside-modifying enzyme from a clinical isolate of Acinetobacter. J Gen Microbiol. 1979 Jan;110(1):239–241. doi: 10.1099/00221287-110-1-239. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Moellering R. C., Jr, Kunz L. J. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine (Baltimore) 1977 Mar;56(2):79–97. doi: 10.1097/00005792-197703000-00001. [DOI] [PubMed] [Google Scholar]
- Goldstein F. W., Labigne-Roussel A., Gerbaud G., Carlier C., Collatz E., Courvalin P. Transferable plasmid-mediated antibiotic resistance in Acinetobacter. Plasmid. 1983 Sep;10(2):138–147. doi: 10.1016/0147-619x(83)90066-5. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Hinchliffe E., Vivian A. Naturally occurring plasmids in Acinetobacter calcoaceticus: a P class R factor of restricted host range. J Gen Microbiol. 1980 Jan;116(1):75–80. doi: 10.1099/00221287-116-1-75. [DOI] [PubMed] [Google Scholar]
- Ingram L. C., Richmond M. H., Sykes R. B. Molecular characterization of the R factors implicated in the carbenicillin resistance of a sequence of Pseudomonas aeruginosa strains isolated from burns. Antimicrob Agents Chemother. 1973 Feb;3(2):279–288. doi: 10.1128/aac.3.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- Le Goffic F., Martel A. Méthode dètude des enzymes inactivant les antibiotiques. Etude de la résistance de Moraxella à la tobramycine la kanamycine et au B.B.K.8. Nouv Presse Med. 1974 Apr 27;3(0):55–56. [PubMed] [Google Scholar]
- Lederberg E. M. Plasmid prefix designations registered by the Plasmid Reference Center 1977-1985. Plasmid. 1986 Jan;15(1):57–92. doi: 10.1016/0147-619x(86)90014-4. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi T., Saito T. beta-Lactamase and beta-lactam antibiotics resistance in acinetobacter anitratum (syn: A. calcoaceticus). J Antibiot (Tokyo) 1977 Nov;30(11):969–973. doi: 10.7164/antibiotics.30.969. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Moellering R. C., Jr Aminoglycoside-modifying enzymes among clinical isolates of Acinetobacter calcoaceticus subsp. anitratus (Herellea vaginicola): explanation for high-level aminoglycoside resistance. Antimicrob Agents Chemother. 1979 Feb;15(2):190–199. doi: 10.1128/aac.15.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Moellering R. C., Jr Evidence of plasmid-mediated production of aminoglycoside-modifying enzymes not previously described in Acinetobacter. Antimicrob Agents Chemother. 1980 Jan;17(1):30–36. doi: 10.1128/aac.17.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. Amikacin resistance associated with a plasmid-borne aminoglycoside phosphotransferase in Escherichia coli. Antimicrob Agents Chemother. 1979 Nov;16(5):598–604. doi: 10.1128/aac.16.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin M. H., Lerner S. A. High-level amikacin resistance in Escherichia coli due to phosphorylation and impaired aminoglycoside uptake. Antimicrob Agents Chemother. 1986 Feb;29(2):216–224. doi: 10.1128/aac.29.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Inoue M., Mitsuhashi S., Naganawa H., Kondo S. Enzymatic adenylylation of spectinomycin by Acinetobacter calcoaceticus subsp. anitratus. J Antibiot (Tokyo) 1981 Jul;34(7):869–875. doi: 10.7164/antibiotics.34.869. [DOI] [PubMed] [Google Scholar]
- Singer J. T., van Tuijl J. J., Finnerty W. R. Transformation and mobilization of cloning vectors in Acinetobacter spp. J Bacteriol. 1986 Jan;165(1):301–303. doi: 10.1128/jb.165.1.301-303.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner K. J., Vivian A. RP4-mediated conjugation in Acinetobacter calcoaceticus. J Gen Microbiol. 1976 Apr;93(2):355–360. doi: 10.1099/00221287-93-2-355. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Evolution and transfer of aminoglycoside resistance genes under natural conditions. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):93–102. doi: 10.1093/jac/18.supplement_c.93. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Gerbaud G., Lambert T., Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985 Dec 16;4(13A):3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. A., Tenover F. C., Gootz T. D., Gordon K. P., Plorde J. J. Development of two DNA probes for differentiating the structural genes of subclasses I and II of the aminoglycoside-modifying enzyme 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1985 May;27(5):739–744. doi: 10.1128/aac.27.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]