Abstract
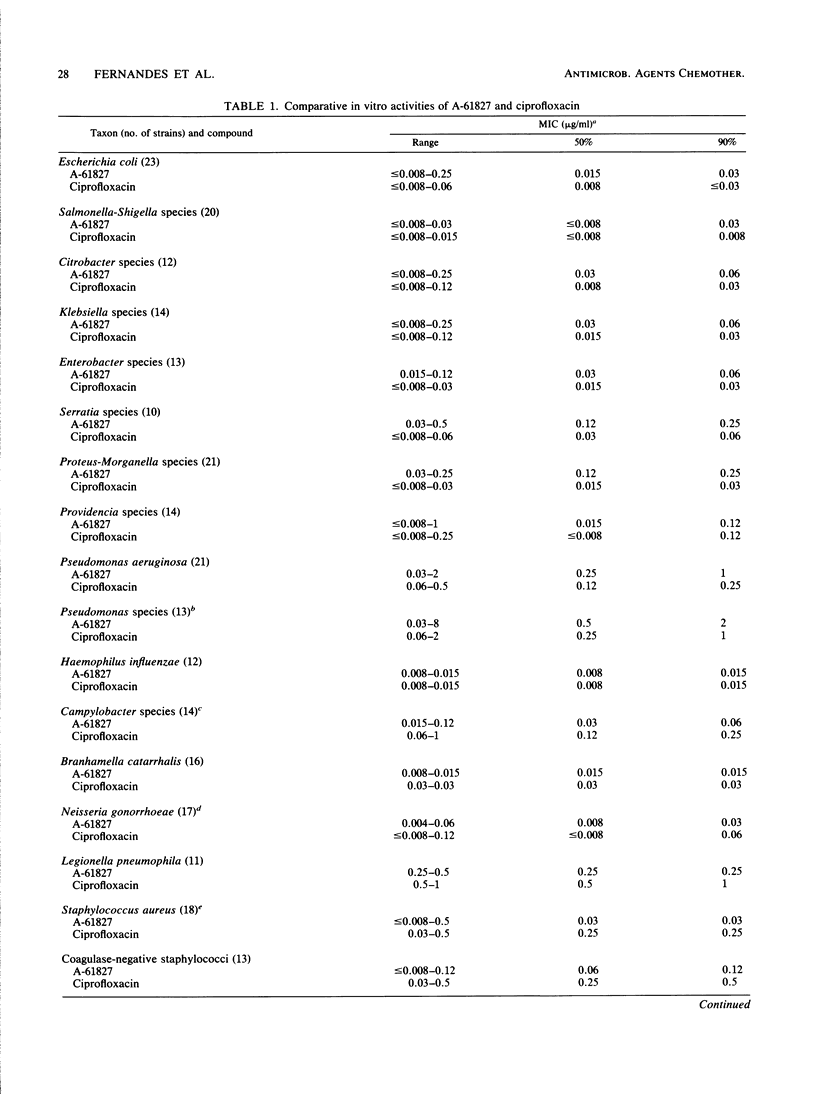
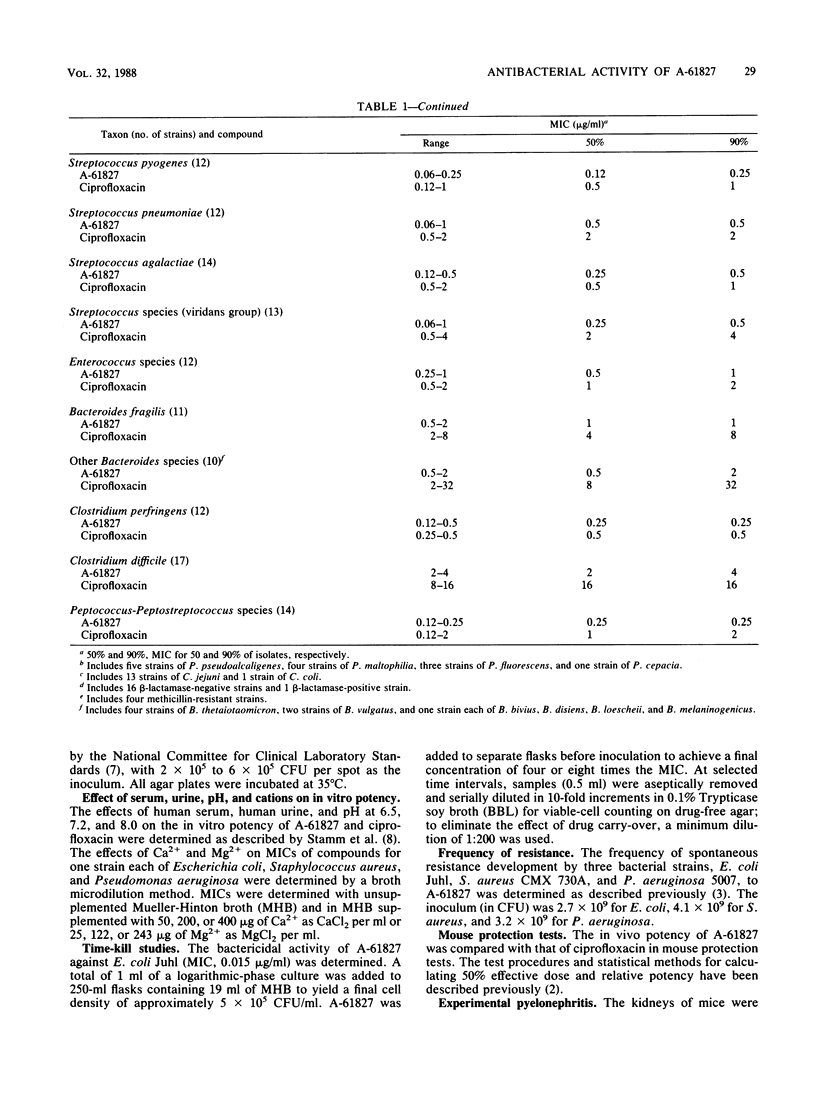
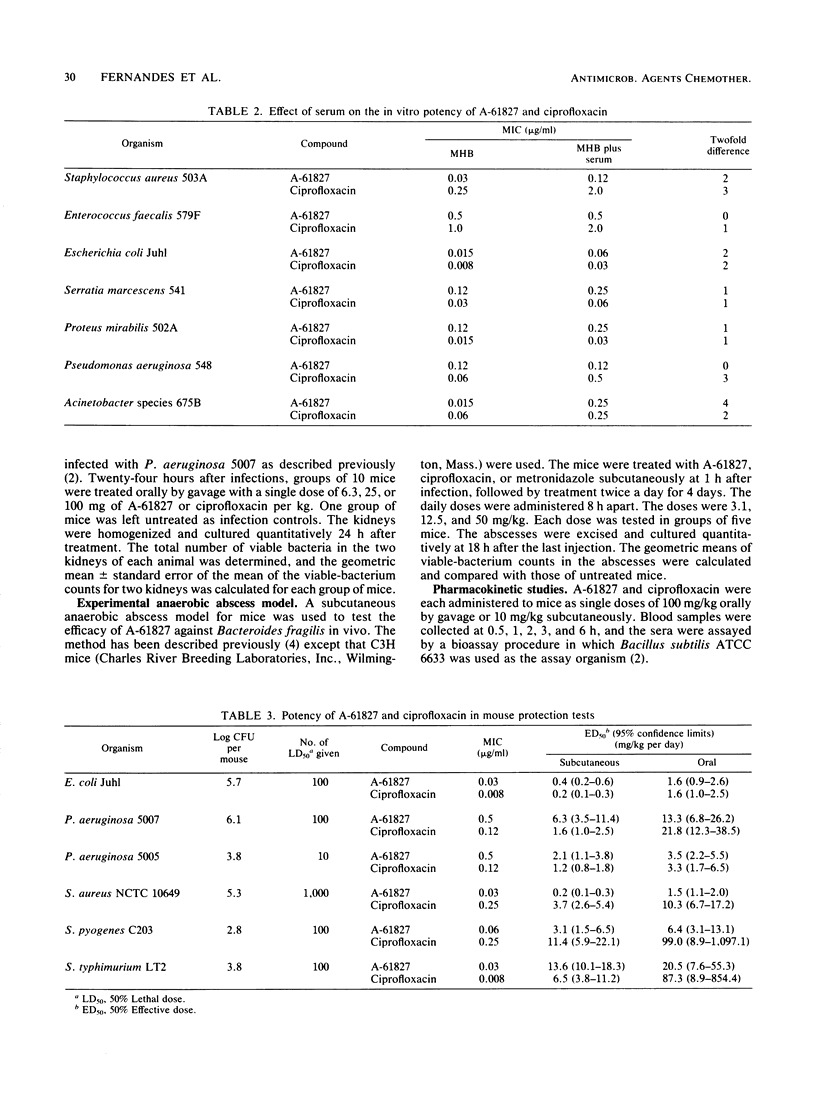
A-61827 (A-60969 is the hydrochloric salt of A-61827) is a new aryl-fluoronaphthyridine which is active against aerobic and anaerobic bacteria. The MICs of A-61827 for 90% of strains (MIC90) of staphylococci and streptococci were less than or equal to 1 microgram/ml and were generally 1 to 4 twofold dilutions less than those of ciprofloxacin for these bacteria. The MIC90S of A-61827 for members of the family Enterobacteriaceae and Pseudomonas aeruginosa were also less than or equal to 1 microgram/ml. Ciprofloxacin was 1 to 3 twofold dilutions more active than A-61827 against these gram-negative bacteria. Neisseria gonorrhoeae, Campylobacter jejuni, and Haemophilus influenzae were susceptible to less than 0.06 microgram of A-61827 per ml. The MIC90 of A-61827 for Legionella pneumophila was 0.25 microgram/ml. A-61827 was as potent or 1 to 2 twofold dilutions more potent than ciprofloxacin against these organisms. The MIC90 of A-61827 for all anaerobic bacteria was less than or equal to 4 micrograms/ml compared with less than or equal to 32 micrograms/ml for ciprofloxacin. In mouse protection tests, A-61827 was as active as ciprofloxacin against Escherichia coli, P. aeruginosa, and Salmonella typhimurium and 5 to 10 times more active than ciprofloxacin against Staphylococcus aureus and Streptococcus pyogenes. A-61827 was as active as ciprofloxacin against P. aeruginosa in a mouse pyelonephritis model and more active than ciprofloxacin and metronidazole in a mouse Bacteroides fragilis abscess model. After oral administration of 100 mg/kg to mice, the peak concentrations of A-61827 and ciprofloxacin in serum were 2.3 and 2.4 micrograms/ml and the half-lives in serum were 3.9 and 1.2 h, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fernandes P. B., Chu D. T., Bower R. R., Jarvis K. P., Ramer N. R., Shipkowitz N. In vivo evaluation of A-56619 (difloxacin) and A-56620: new aryl-fluoroquinolones. Antimicrob Agents Chemother. 1986 Feb;29(2):201–208. doi: 10.1128/aac.29.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Hanson C. W., Stamm J. M., Vojtko C., Shipkowitz N. L., St Martin E. The frequency of in-vitro resistance development to fluoroquinolones and the use of a murine pyelonephritis model to demonstrate selection of resistance in vivo. J Antimicrob Chemother. 1987 Apr;19(4):449–465. doi: 10.1093/jac/19.4.449. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Shipkowitz N., Bower R. R., Jarvis K. P., Weisz J., Chu D. T. In-vitro and in-vivo potency of five new fluoroquinolones against anaerobic bacteria. J Antimicrob Chemother. 1986 Dec;18(6):693–701. doi: 10.1093/jac/18.6.693. [DOI] [PubMed] [Google Scholar]
- King A., Phillips I. The comparative in-vitro activity of eight newer quinolones and nalidixic acid. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):1–20. doi: 10.1093/jac/18.supplement_d.1. [DOI] [PubMed] [Google Scholar]
- Stamm J. M., Hanson C. W., Chu D. T., Bailer R., Vojtko C., Fernandes P. B. In vitro evaluation of A-56619 (difloxacin) and A-56620: new aryl-fluoroquinolones. Antimicrob Agents Chemother. 1986 Feb;29(2):193–200. doi: 10.1128/aac.29.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]


