Abstract
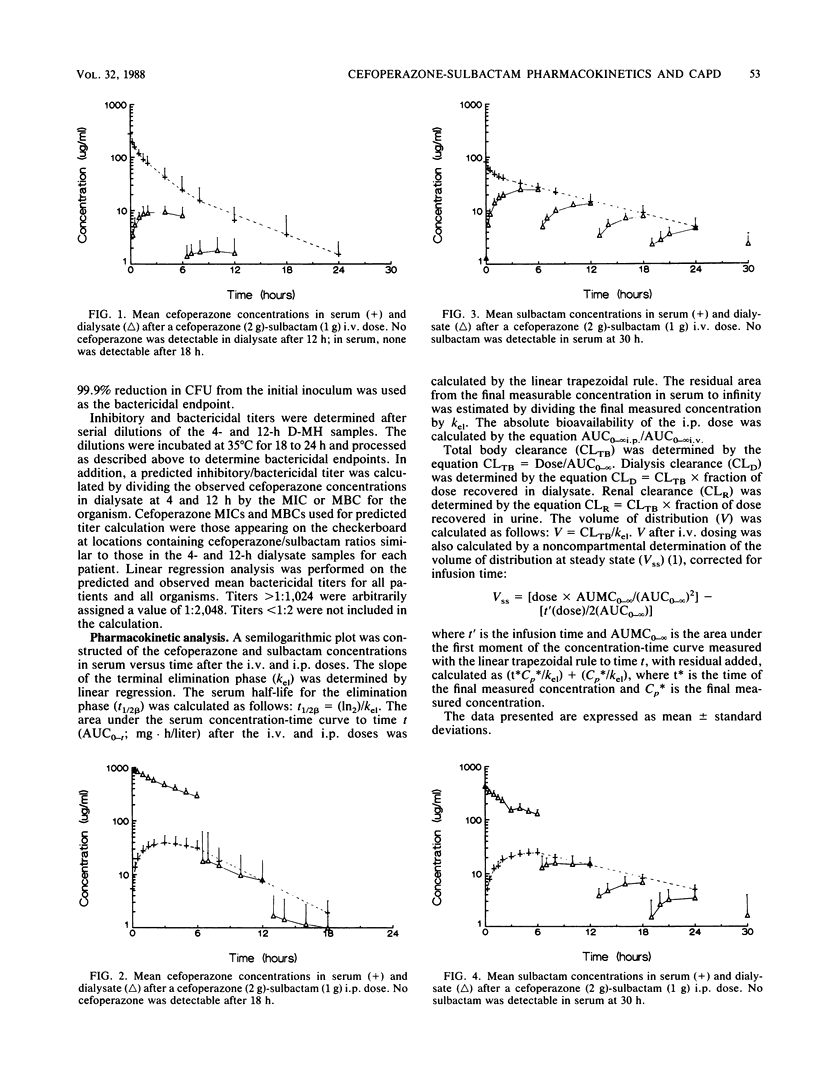
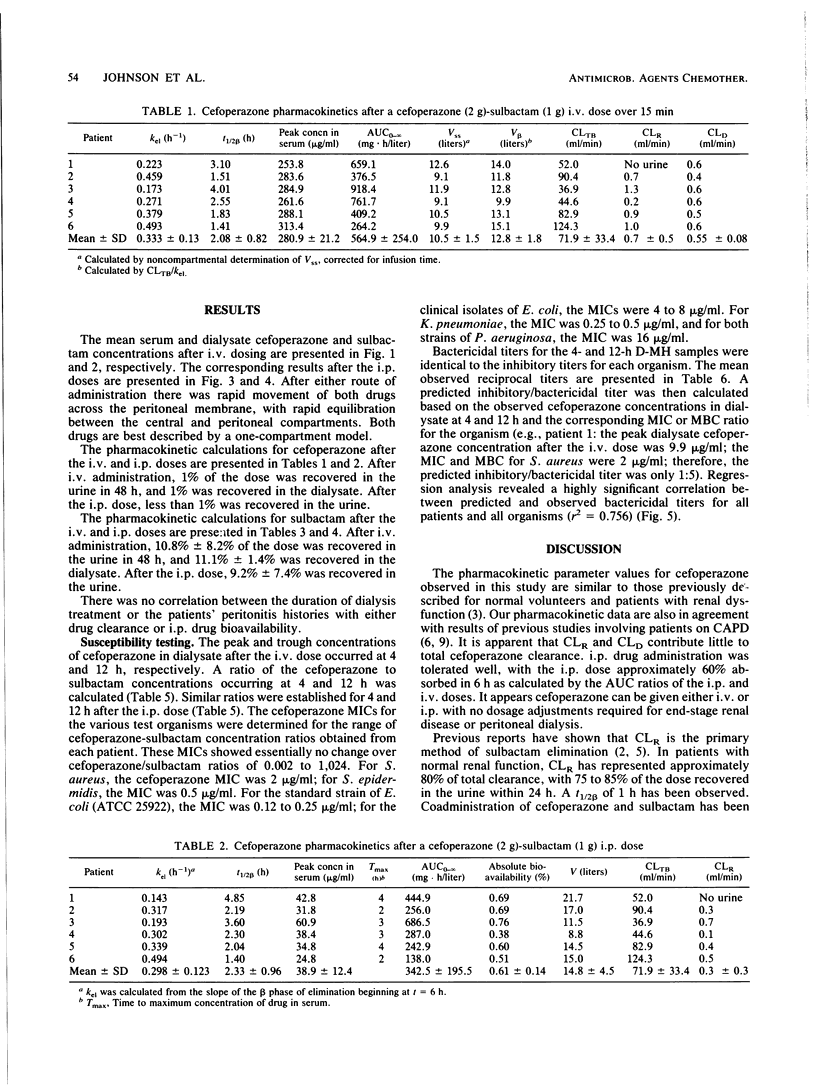
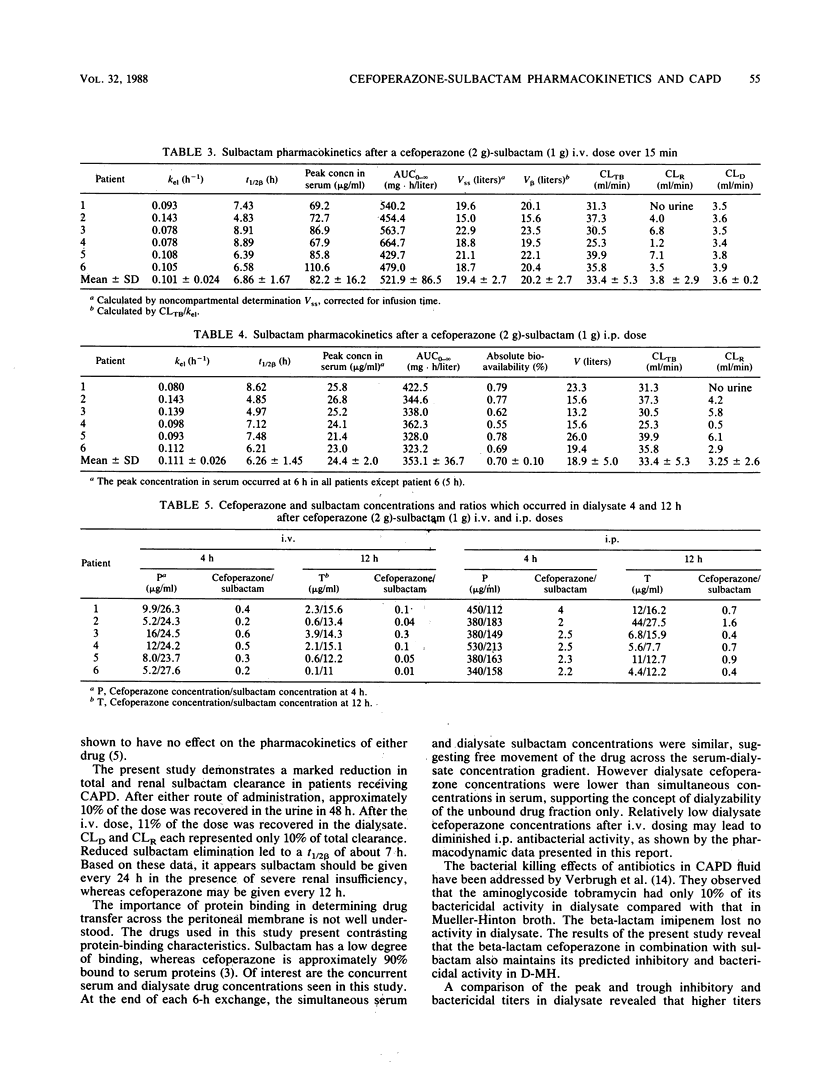
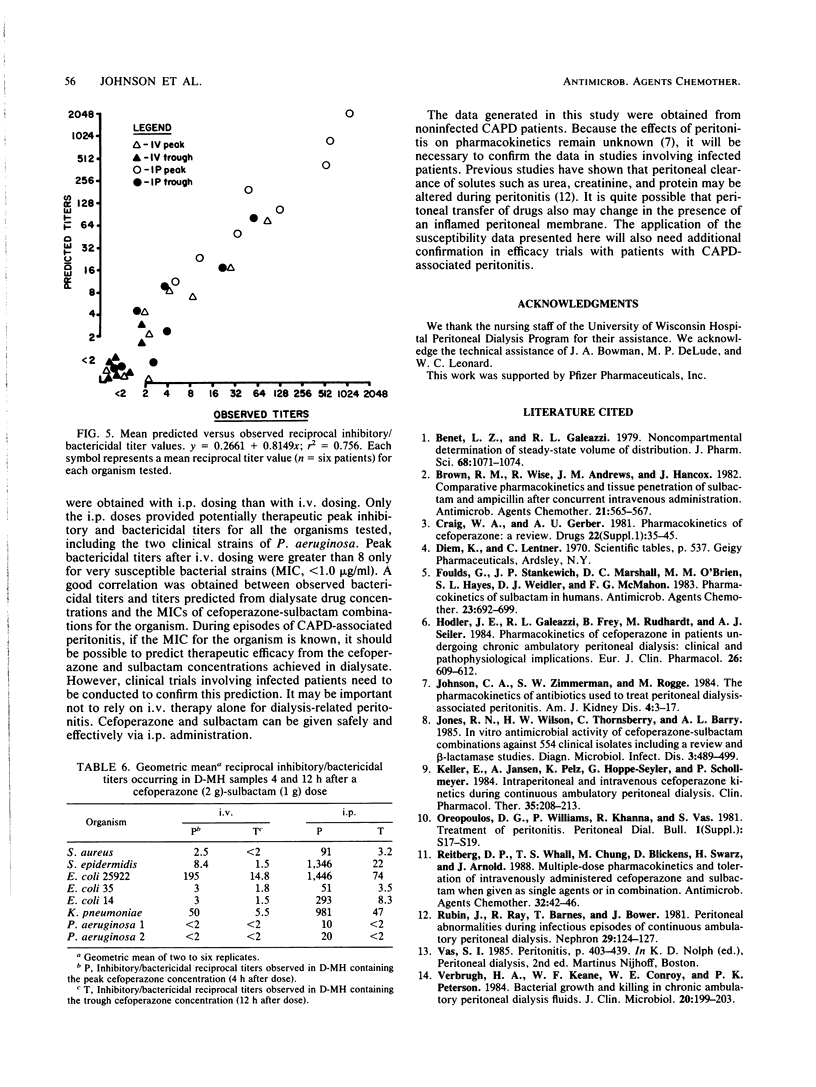
This study was conducted to determine the pharmacokinetics of the fixed combination antibiotic cefoperazone-sulbactam in patients receiving continuous ambulatory peritoneal dialysis (CAPD). In addition, the pharmacodynamic profile of this combination was determined by the use of mean bactericidal titers against selected bacterial strains. Six noninfected CAPD patients were given a fixed dose of cefoperazone (2 g) and sulbactam (1 g) either intravenously or intraperitoneally over 10 min in a randomized, two-way crossover fashion. The mean peak cefoperazone concentration in serum after intravenous administration was 280.9 micrograms/ml. The mean peak concentration in serum after intraperitoneal cefoperazone administration was 38.9 micrograms/ml and occurred 2 to 4 h postdose. The mean peak sulbactam concentration in serum after intravenous administration was 82.2 micrograms/ml. The mean peak concentration in serum after intraperitoneal sulbactam administration was 24.4 micrograms/ml and occurred at 6 h. The absolute bioavailability of the intraperitoneal dose was 61% for cefoperazone and 70% for sulbactam. Cefoperazone total body and renal clearances were unaffected by renal failure and dialysis. However, both clearance values for sulbactam were reduced markedly. Only intraperitoneal dosing provided peak inhibitory and bactericidal titers in dialysate for all organisms tested. Intravenous dosing provided satisfactory dialysate titers only for very susceptible bacterial strains. End-stage renal disease and CAPD do not alter cefoperazone pharmacokinetics; however, sulbactam dosing may need to be adjusted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Wise R., Andrews J. M., Hancox J. Comparative pharmacokinetics and tissue penetration of sulbactam and ampicillin after concurrent intravenous administration. Antimicrob Agents Chemother. 1982 Apr;21(4):565–567. doi: 10.1128/aac.21.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A., Gerber A. U. Pharmacokinetics of cefoperazone: a review. Drugs. 1981;22 (Suppl 1):35–45. doi: 10.2165/00003495-198100221-00010. [DOI] [PubMed] [Google Scholar]
- Foulds G., Stankewich J. P., Marshall D. C., O'Brien M. M., Hayes S. L., Weidler D. J., McMahon F. G. Pharmacokinetics of sulbactam in humans. Antimicrob Agents Chemother. 1983 May;23(5):692–699. doi: 10.1128/aac.23.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodler J. E., Galeazzi R. L., Frey B., Rudhardt M., Seiler A. J. Pharmacokinetics of cefoperazone in patients undergoing chronic ambulatory peritoneal dialysis: clinical and pathophysiological implications. Eur J Clin Pharmacol. 1984;26(5):609–612. doi: 10.1007/BF00543494. [DOI] [PubMed] [Google Scholar]
- Johnson C. A., Zimmerman S. W., Rogge M. The pharmacokinetics of antibiotics used to treat peritoneal dialysis-associated peritonitis. Am J Kidney Dis. 1984 Jul;4(1):3–17. doi: 10.1016/s0272-6386(84)80020-7. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Wilson H. W., Thornsberry C., Barry A. L. In vitro antimicrobial activity of cefoperazone-sulbactam combinations against 554 clinical isolates including a review and beta-lactamase studies. Diagn Microbiol Infect Dis. 1985 Nov;3(6):489–499. doi: 10.1016/s0732-8893(85)80005-5. [DOI] [PubMed] [Google Scholar]
- Keller E., Jansen A., Pelz K., Hoppe-Seyler G., Schollmeyer P. Intraperitoneal and intravenous cefoperazone kinetics during continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther. 1984 Feb;35(2):208–213. doi: 10.1038/clpt.1984.28. [DOI] [PubMed] [Google Scholar]
- Reitberg D. P., Whall T. J., Chung M., Blickens D., Swarz H., Arnold J. Multiple-dose pharmacokinetics and toleration of intravenously administered cefoperazone and sulbactam when given as single agents or in combination. Antimicrob Agents Chemother. 1988 Jan;32(1):42–46. doi: 10.1128/aac.32.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J., Ray R., Barnes T., Bower J. Peritoneal abnormalities during infectious episodes of continuous ambulatory peritoneal dialysis. Nephron. 1981;29(3-4):124–127. doi: 10.1159/000182328. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Conroy W. E., Peterson P. K. Bacterial growth and killing in chronic ambulatory peritoneal dialysis fluids. J Clin Microbiol. 1984 Aug;20(2):199–203. doi: 10.1128/jcm.20.2.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


