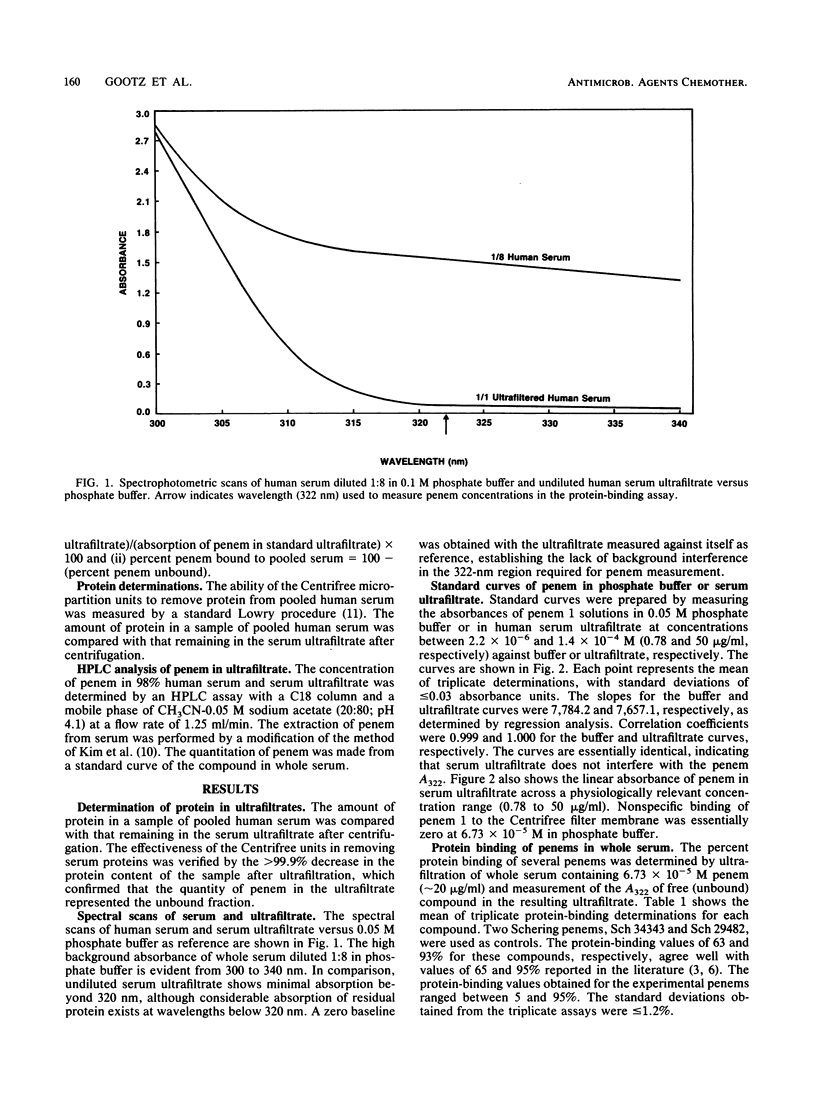
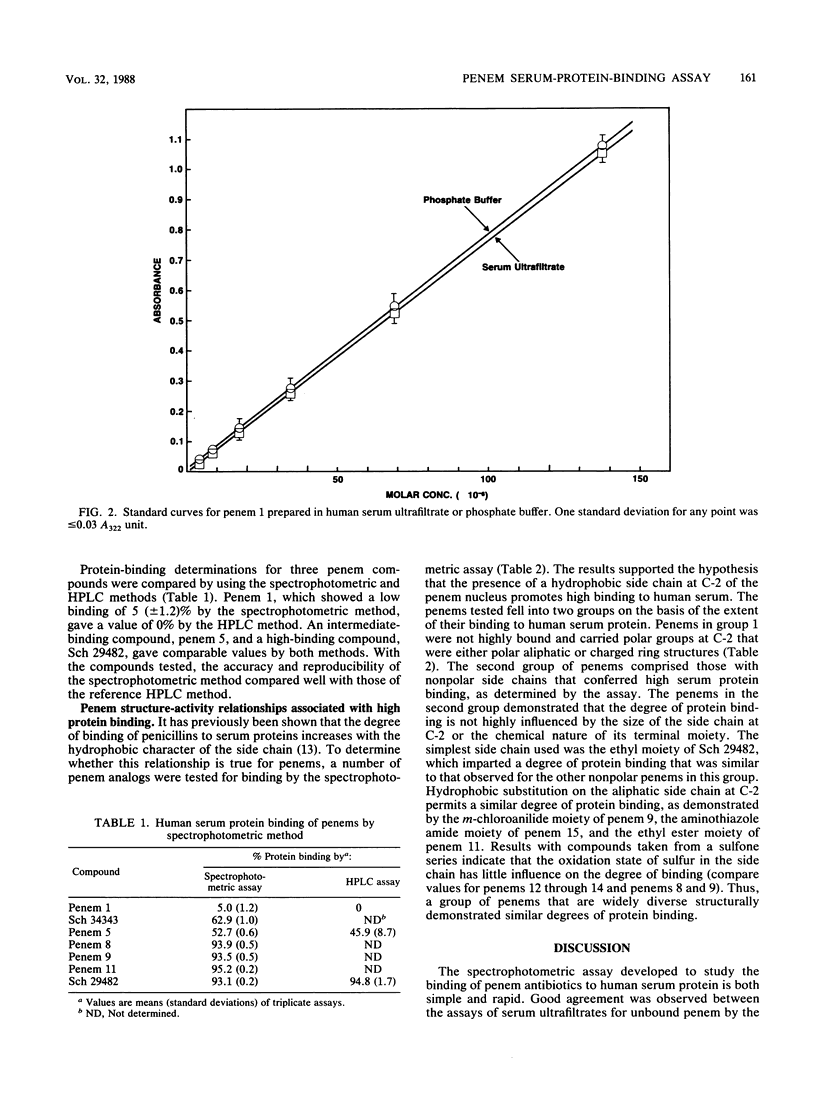
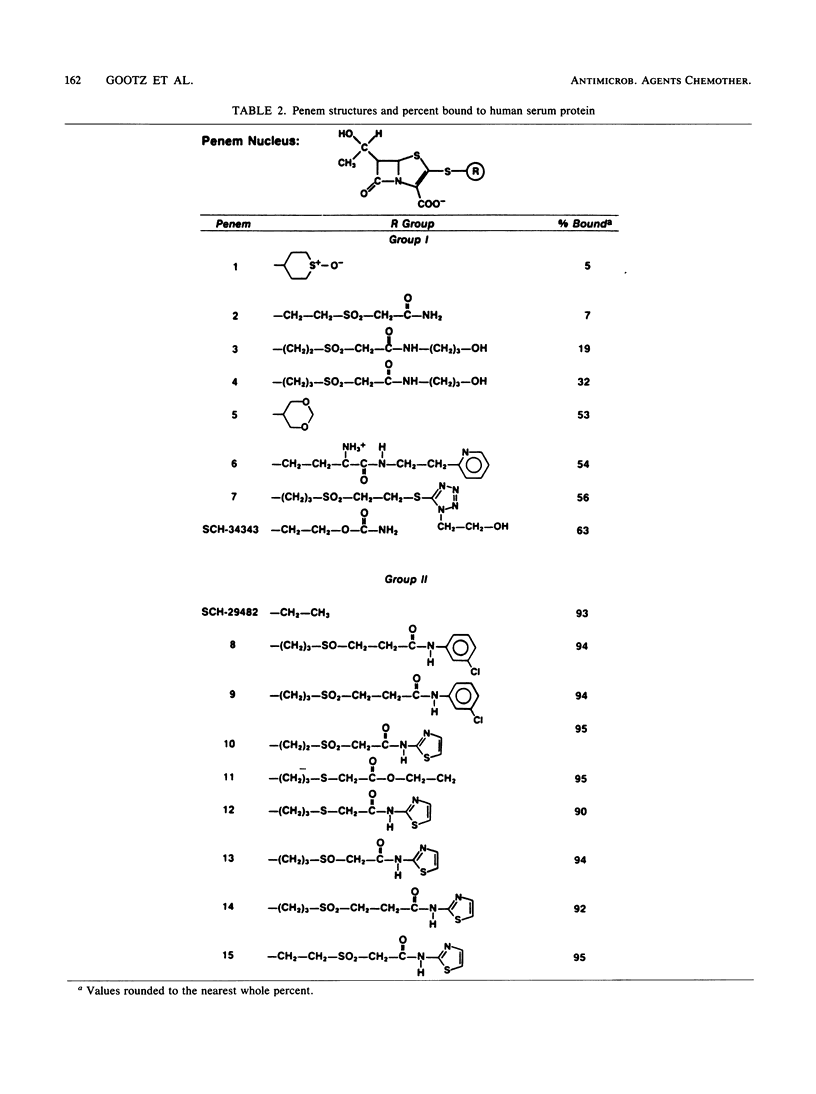
Abstract
The binding of antibiotics to plasma (serum) proteins through hydrogen bonding can significantly influence the biological characteristics of these drugs. A rapid spectrophotometric assay has been developed that measures the level of free (unbound) penem antibiotic in serum ultrafiltrates. Whole human serum was adjusted to a standard concentration of antibiotic and then filtered by centrifugation through a Centrifree (Amicon Corp., Lexington, Mass.) filter that retained greater than 99.9% of serum protein. The degree of penem protein binding was determined spectrophotometrically by measuring the level of unbound drug in the ultrafiltrate at 322 nm. At this wavelength, no interfering absorption from residual protein was detected in the ultrafiltrate, and penem absorption was linear over a wide concentration range. The method gave protein-binding values comparable to those obtained by a high-pressure liquid chromatography assay but was more rapid, since it did not require solvent extraction and high-pressure liquid chromatography calibration procedures. The spectrophotometric assay has been used to assay over 100 penems to determine the structure-activity relationships that are involved with the high serum protein binding of these agents. As with penicillins and some cephalosporins, the nonpolar nature of the penem side chain at the C-2 position strongly influenced the degree of penem binding to serum proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barza M. Principles of tissue penetration of antibiotics. J Antimicrob Chemother. 1981 Nov;8 (Suppl 100):7–28. doi: 10.1093/jac/8.suppl_c.7. [DOI] [PubMed] [Google Scholar]
- Bird A. E., Marshall A. C. Correlation of serum binding of penicillins with partition coefficients. Biochem Pharmacol. 1967 Dec;16(12):2275–2290. doi: 10.1016/0006-2952(67)90215-8. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Wise R., Andrews J. M. Sch 29482--a novel penem antibiotic: an in-vitro comparison of its activity with other beta-lactams. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):17–23. doi: 10.1093/jac/9.suppl_c.17. [DOI] [PubMed] [Google Scholar]
- Bruderlein H., Daniel R., Perras D., DiPaola A., Fideliá A., Belleau B. An investigation of the destruction of the beta-lactam ring of penems by the albumin drug-binding site. Can J Biochem. 1981 Oct;59(10):857–866. doi: 10.1139/o81-118. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Reiszner E., Moellering R. C., Jr In vitro activity of Sch 34343 against enterococci and other gram-positive bacteria. Antimicrob Agents Chemother. 1985 Jan;27(1):28–32. doi: 10.1128/aac.27.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay C. D., Wise R., Allcock J. E., Durham S. R. The tissue penetration, as measured by a blister technique, and pharmacokinetics of cefsulodin compared with carbenicillin and ticarcillin. J Antimicrob Chemother. 1981 Jun;7(6):637–642. doi: 10.1093/jac/7.6.637. [DOI] [PubMed] [Google Scholar]
- Halver B. Rapid method for determining ultrafiltrable calcium in serum. Clin Chem. 1972 Dec;18(12):1488–1492. [PubMed] [Google Scholar]
- Jerkunica I., Sophianopoulos J., Sgoutas D. Improved ultrafiltration method for determining unbound cortisol in plasma. Clin Chem. 1980 Nov;26(12):1734–1737. [PubMed] [Google Scholar]
- Kim H., Oden E., Lin C. High-pressure liquid chromatographic method for determination of Sch 29482 in human serum. Antimicrob Agents Chemother. 1982 Nov;22(5):848–851. doi: 10.1128/aac.22.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Callaghan C. H. Irreversible effects of serum proteins on beta-lactam antibiotics. Antimicrob Agents Chemother. 1978 Apr;13(4):628–633. doi: 10.1128/aac.13.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. O., Power D. M., Robinson C., Davies J. V. Ion binding of penicillins to proteins. Biochim Biophys Acta. 1970 Sep 22;215(3):491–502. doi: 10.1016/0304-4165(70)90099-1. [DOI] [PubMed] [Google Scholar]
- Shah V. P., Wallace S. M., Riegelman S. Microultrafiltration technique for drug-protein binding determination in plasma. J Pharm Sci. 1974 Sep;63(9):1364–1367. doi: 10.1002/jps.2600630905. [DOI] [PubMed] [Google Scholar]
- Singhvi S. M., Heald A. F., Gadebusch H. H., Resnick M. E., Difazio L. T., Leitz M. A. Human serum protein binding of cephalosporin antibiotics in vitro. J Lab Clin Med. 1977 Feb;89(2):414–420. [PubMed] [Google Scholar]
- Sophianopoulos J. A., Durham S. J., Sophianopoulos A. J., Ragsdale H. L., Cropper W. P., Jr Ultrafiltration is theoretically equivalent to equilibrium dialysis but much simpler to carry out. Arch Biochem Biophys. 1978 Apr 15;187(1):132–137. doi: 10.1016/0003-9861(78)90015-2. [DOI] [PubMed] [Google Scholar]
- Whitlam J. B., Brown K. F. Ultrafiltration in serum protein binding determinations. J Pharm Sci. 1981 Feb;70(2):146–150. doi: 10.1002/jps.2600700208. [DOI] [PubMed] [Google Scholar]
- Wise R. Protein binding of beta-lactams: the effects on activity and pharmacology particularly tissue penetration. I. J Antimicrob Chemother. 1983 Jul;12(1):1–18. doi: 10.1093/jac/12.1.1. [DOI] [PubMed] [Google Scholar]


