Abstract
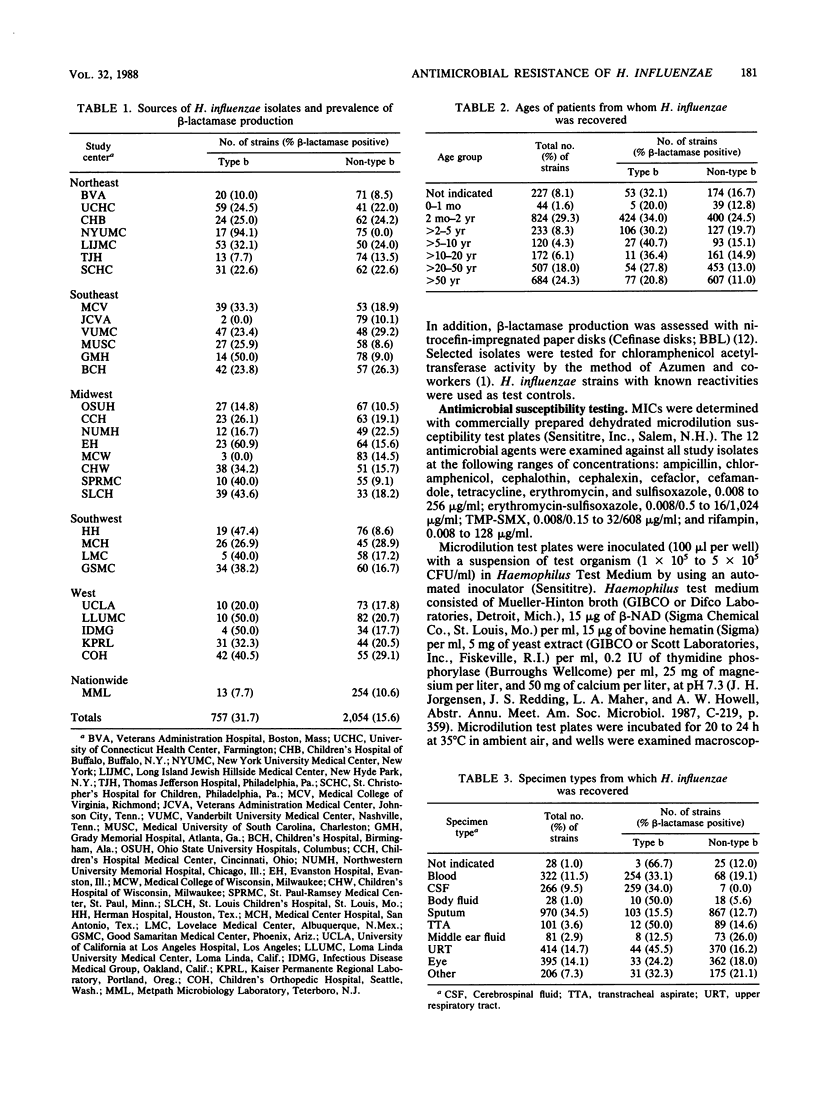
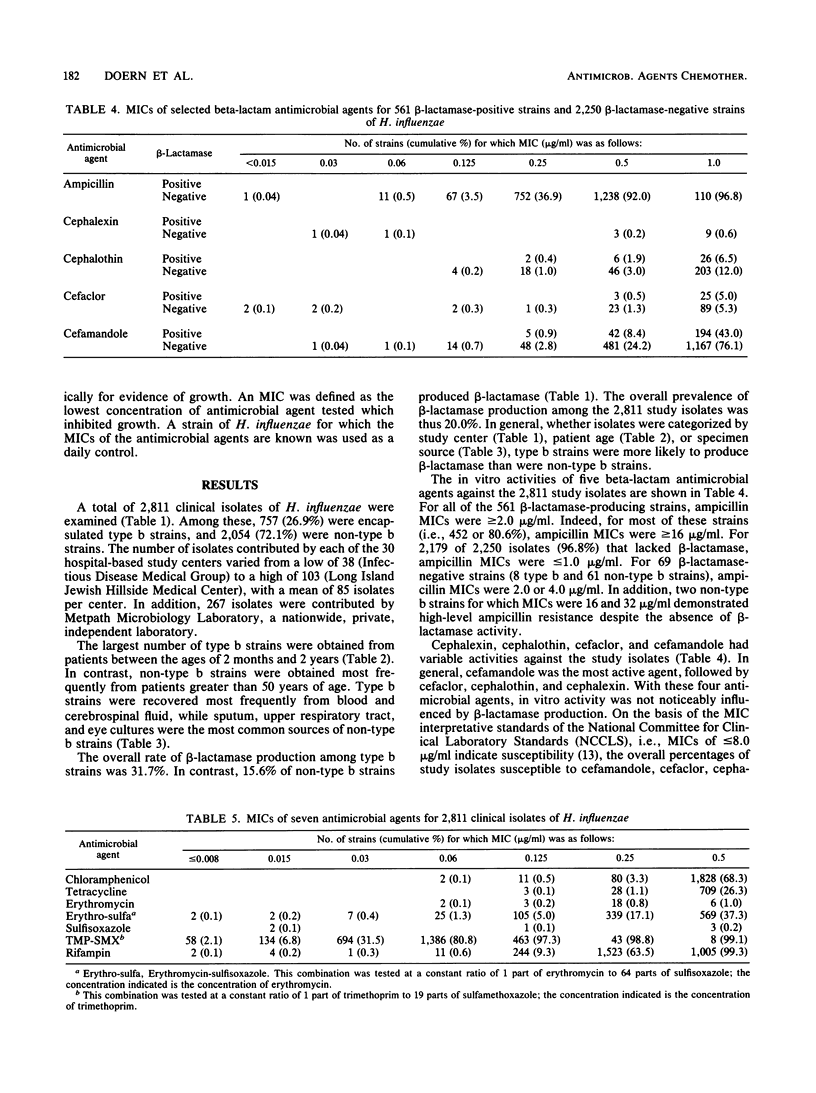
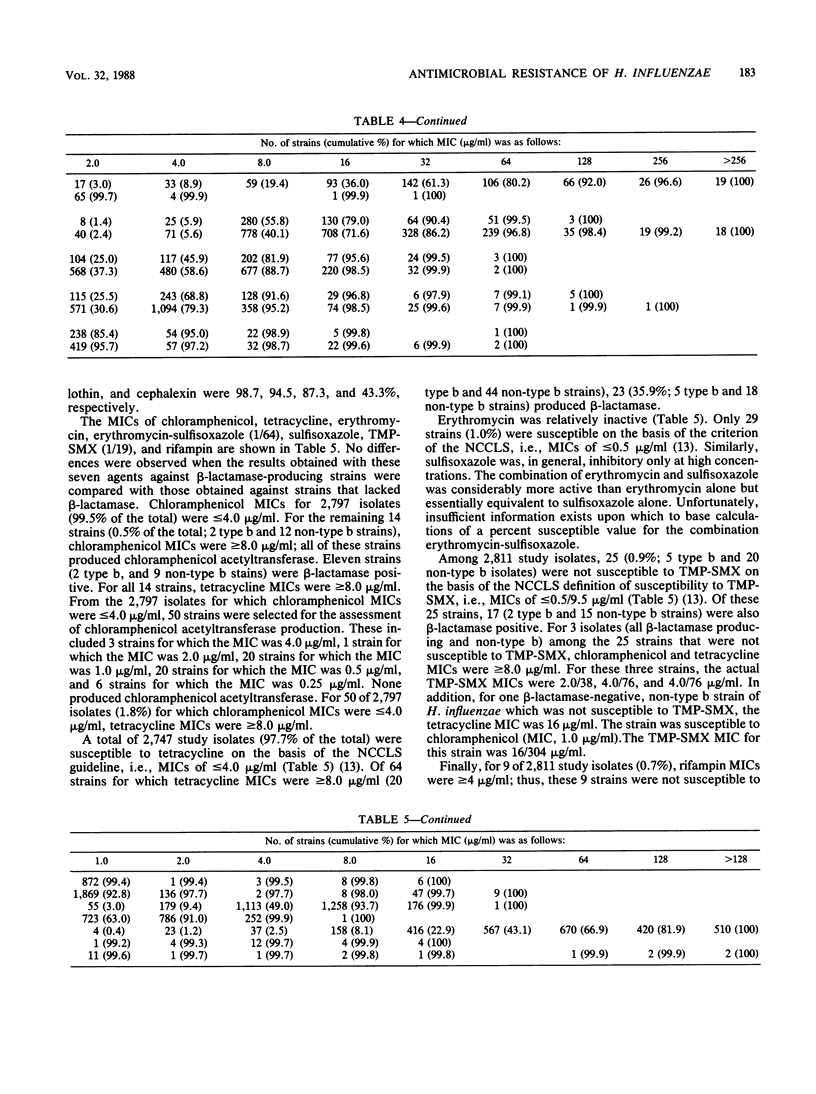
A total of 2,811 clinical isolates of Haemophilus influenzae were obtained during 1986 from 30 medical centers and one nationwide private independent laboratory in the United States. Among these, 757 (26.9%) were type b strains. The overall rate of beta-lactamase-mediated ampicillin resistance was 20.0%. Type b strains were approximately twice as likely as non-type b strains to produce beta-lactamase (31.7 versus 15.6%). The MICs of 12 antimicrobial agents were determined for all isolates. Ampicillin resistance among strains that lacked beta-lactamase activity was extremely uncommon (0.1%). Percentages of study isolates susceptible to cefamandole, cefaclor, cephalothin, and cephalexin were 98.7, 94.5, 87.3, and 43.3%, respectively. For 14 strains (0.5% of the total), chloramphenicol MICs were greater than or equal to 8.0 micrograms, and thus the strains were considered resistant. All of these resistant strains produced chloramphenicol acetyltransferase. In addition, all 14 strains were resistant to tetracycline; 11 produced beta-lactamase. The percentage of isolates susceptible to tetracycline was 97.7%. In contrast, erythromycin and sulfisoxazole were relatively inactive. The combination of erythromycin-sulfisoxazole (1/64) was more active than erythromycin alone but essentially equivalent in activity to sulfisoxazole alone. Finally, small numbers of clinical isolates of H. influenzae were resistant to trimethoprim-sulfamethoxazole and rifampin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azemun P., Stull T., Roberts M., Smith A. L. Rapid detection of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1981 Aug;20(2):168–170. doi: 10.1128/aac.20.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Mendelman P. M., Levy J., Stull T. L., Smith A. L. A permeability barrier as a mechanism of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Jan;27(1):46–54. doi: 10.1128/aac.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J., Garcia-Tornel S., Sanfeliu I. Susceptibility studies of multiply resistant Haemophilus influenzae isolated from pediatric patients and contacts. Antimicrob Agents Chemother. 1984 Jun;25(6):706–709. doi: 10.1128/aac.25.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Daum G. S., Tubert T. A. Ampicillin disk diffusion susceptibility testing of Haemophilus influenzae. J Clin Microbiol. 1987 Sep;25(9):1675–1678. doi: 10.1128/jcm.25.9.1675-1678.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Daum G. S., Tubert T. A. In vitro chloramphenicol susceptibility testing of Haemophilus influenzae: disk diffusion procedures and assays for chloramphenicol acetyltransferase. J Clin Microbiol. 1987 Aug;25(8):1453–1455. doi: 10.1128/jcm.25.8.1453-1455.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Jorgensen J. H., Thornsberry C., Preston D. A. Prevalence of antimicrobial resistance among clinical isolates of Haemophilus influenzae: a collaborative study. Diagn Microbiol Infect Dis. 1986 Feb;4(2):95–107. doi: 10.1016/0732-8893(86)90143-4. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Krause P. J., Owens N. J., Nightingale C. H., Klimek J. J., Lehmann W. B., Quintiliani R. Penetration of amoxicillin, cefaclor, erythromycin-sulfisoxazole, and trimethoprim-sulfamethoxazole into the middle ear fluid of patients with chronic serous otitis media. J Infect Dis. 1982 Jun;145(6):815–821. doi: 10.1093/infdis/145.6.815. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., Levesque R., Jacoby G. A. An animal source for the ROB-1 beta-lactamase of Haemophilus influenzae type b. Antimicrob Agents Chemother. 1986 Feb;29(2):212–215. doi: 10.1128/aac.29.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Stull T. L., Rubens C. E., Mack K. D., Smith A. L. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984 Aug;26(2):235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery K., Raymundo L., Jr, Drew W. L. Chromogenic cephalosporin spot test to detect beta-lactamase in clinically significant bacteria. J Clin Microbiol. 1979 Feb;9(2):205–207. doi: 10.1128/jcm.9.2.205-207.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Mechanism of resistance of an ampicillin-resistant, beta-lactamase-negative clinical isolate of Haemophilus influenzae type b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):747–753. doi: 10.1128/aac.25.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Swenson C. D., Owens L. M., Smith A. L. Characterization of chloramphenicol-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1980 Oct;18(4):610–615. doi: 10.1128/aac.18.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]



