Abstract
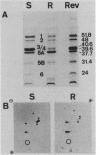
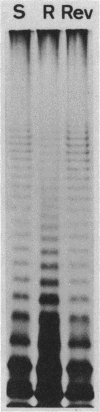
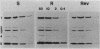
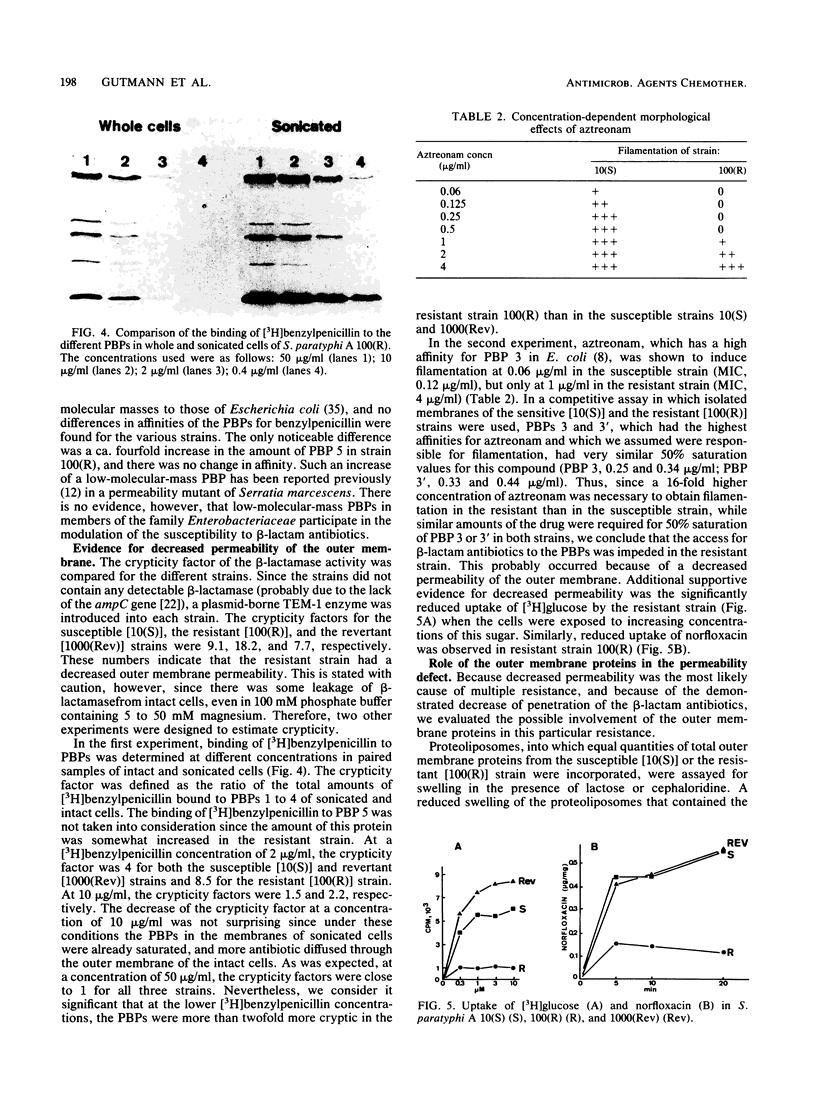
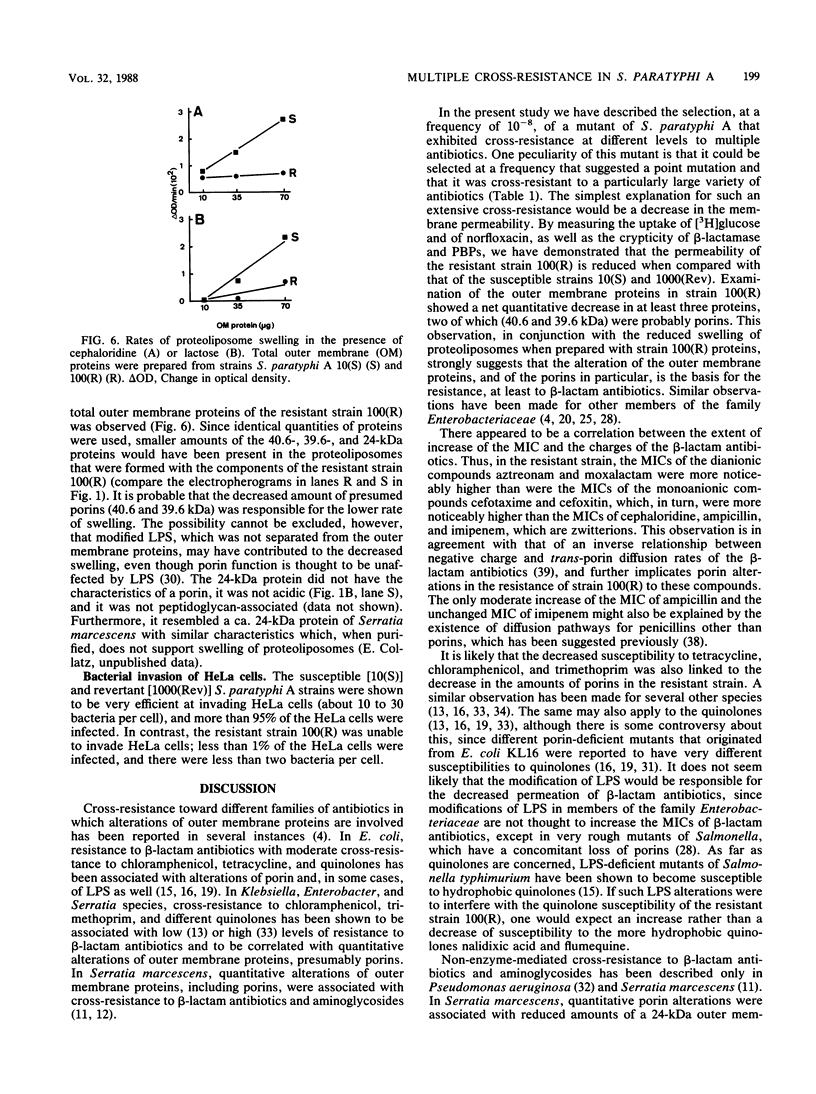
A spontaneous one-step mutant of Salmonella paratyphi A selected on ampicillin showed cross-resistance to all beta-lactam antibiotics except imipenem and to aminoglycosides, chloramphenicol, tetracycline, trimethoprim, and quinolones. It also grew as small colonies. Examination of the cell envelope of the mutant showed a quantitative decrease in three major outer membrane proteins of 40.6, 39.6 (presumably porins), and 24 kilodaltons and quantitative as well as qualitative modifications in the ladder pattern of lipopolysaccharide. Direct evidence for decreased permeability in the mutant included reduced uptake of [3H]glucose and norfloxacin, reduced accessibility of aztreonam and benzylpenicillin to penicillin-binding proteins in whole cells, and decreased diffusion of lactose and cephaloridine into proteoliposomes that were reconstituted with outer membrane proteins from the mutant. There was also loss of invasiveness of the mutant into HeLa cells. We assume that a pleiotropic mutation was responsible for multiple alterations in the outer membrane components of the resistant mutant of S. paratyphi A.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Sonntag I., Henning U. Cloning and expression in Escherichia coli K-12 of the genes for major outer membrane protein OmpA from Shigella dysenteriae, Enterobacter aerogenes, and Serratia marcescens. J Bacteriol. 1982 Jan;149(1):145–150. doi: 10.1128/jb.149.1.145-150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Chai T. J. New major outer membrane proteins found in an Escherichia coli tolF mutant resistant to bacteriophage TuIb. J Bacteriol. 1978 Mar;133(3):1478–1483. doi: 10.1128/jb.133.3.1478-1483.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A. M., Levy S. B. Gene in the major cotransduction gap of the Escherichia coli K-12 linkage map required for the expression of chromosomal resistance to tetracycline and other antibiotics. J Bacteriol. 1983 Aug;155(2):541–548. doi: 10.1128/jb.155.2.541-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Sykes R. B. Mode of action of azthreonam. Antimicrob Agents Chemother. 1982 Jun;21(6):950–956. doi: 10.1128/aac.21.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Washington O., Gemski P., Formal S. B. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J Infect Dis. 1973 Jul;128(1):69–75. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein F. W., Gutmann L., Williamson R., Collatz E., Acar J. F. In vivo and in vitro emergence of simultaneous resistance to both beta-lactam and aminoglycoside antibiotics in a strain of Serratia marcescens. Ann Microbiol (Paris) 1983 May-Jun;134A(3):329–337. [PubMed] [Google Scholar]
- Gutmann L., Chabbert Y. A. Different mechanisms of resistance to latamoxef (moxalactam) in Serratia marcescens. J Antimicrob Chemother. 1984 Jan;13(1):15–22. doi: 10.1093/jac/13.1.15. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981 Apr;32(1):137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A., Chabbert Y. A., Semonin O. Role of porin proteins OmpF and OmpC in the permeation of beta-lactams. Antimicrob Agents Chemother. 1982 Dec;22(6):942–948. doi: 10.1128/aac.22.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Normark S. Contribution of chromosomal beta-lactamases to beta-lactam resistance in enterobacteria. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S292–S304. doi: 10.1093/clinids/8.supplement_3.s292. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama J., Hiruma R., Yamaguchi A., Sawai T. Identification of porins in outer membrane of Proteus, Morganella, and Providencia spp. and their role in outer membrane permeation of beta-lactams. Antimicrob Agents Chemother. 1987 Mar;31(3):379–384. doi: 10.1128/aac.31.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Moellering R. C., Jr In-vivo acquisition of two different types of aminoglycoside resistance by a single strain of Klebsiella pneumoniae causing severe infection. Ann Intern Med. 1982 Feb;96(2):176–180. doi: 10.7326/0003-4819-96-2-176. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Poole K., Crockford G. W., Hancock R. E. Lipopolysaccharide-free Escherichia coli OmpF and Pseudomonas aeruginosa protein P porins are functionally active in lipid bilayer membranes. J Bacteriol. 1986 Feb;165(2):523–526. doi: 10.1128/jb.165.2.523-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. The effect of altered porin expression in Escherichia coli upon susceptibility to 4-quinolones. J Antimicrob Chemother. 1986 Oct;18(4):547–549. doi: 10.1093/jac/18.4.547. [DOI] [PubMed] [Google Scholar]
- Preheim L. C., Penn R. G., Sanders C. C., Goering R. V., Giger D. K. Emergence of resistance to beta-lactam and aminoglycoside antibiotics during moxalactam therapy of Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 1982 Dec;22(6):1037–1041. doi: 10.1128/aac.22.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Multiply resistant mutants of Enterobacter cloacae selected by beta-lactam antibiotics. Antimicrob Agents Chemother. 1986 Nov;30(5):684–688. doi: 10.1128/aac.30.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. C., McCashion R. N., Lynch W. H. Multiple low-level antibiotic resistance in Aeromonas salmonicida. Antimicrob Agents Chemother. 1986 Jun;29(6):992–996. doi: 10.1128/aac.29.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Tomiyama N., Hiruma R., Sawai T. Difference in pathway of Escherichia coli outer membrane permeation between penicillins and cephalosporins. FEBS Lett. 1985 Feb 11;181(1):143–148. doi: 10.1016/0014-5793(85)81130-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]