Abstract
Aim: To characterise the change in serum and urinary bone markers in the early postnatal period, and to assess the effect of systemic corticosteroid on bone metabolism in preterm infants.
Methods: Bone formation was quantified by measurement of serum concentrations of bone specific alkaline phosphatase (BALP) and osteocalcin. Bone resorption was measured by monitoring creatinine adjusted urinary deoxypyridinoline (Dpd) concentration. Blood and urinary samples were collected from corticosteroid treated infants (n = 19) immediately before the start (Td-pre), three weeks after the start (Td-end), and two (Td-post2) and four weeks (Td-post4) after the end of the dexamethasone course. Untreated patients (n = 30) had specimens taken at week 3 (Twk-3), 6 (Twk-6), 8 (Twk-8), and 10 (Twk-10) of postnatal age.
Results: Serum concentrations of BALP and osteocalcin at Td-end were significantly lower than pretreatment levels and the levels at the corresponding time point (Twk-6) of the non-treatment group. In contrast, urinary Dpd concentration at Td-end was not significantly decreased compared with the pretreatment level. However, it was significantly lower than the urinary Dpd concentration at Twk-6 of the non-treatment group. The rate of increase in lower leg length was significantly higher in the non-treatment group between weeks 3 and 6 than in the corresponding period during dexamethasone treatment in the corticosteroid group.
Conclusion: Systemic corticosteroid causes appreciable suppression of serum BALP and osteocalcin and, to a lesser extent, urinary Dpd. The results suggest that corticosteroid inhibits bone growth mainly by decreasing bone formation.
Full Text
The Full Text of this article is available as a PDF (119.5 KB).
Figure 1 .
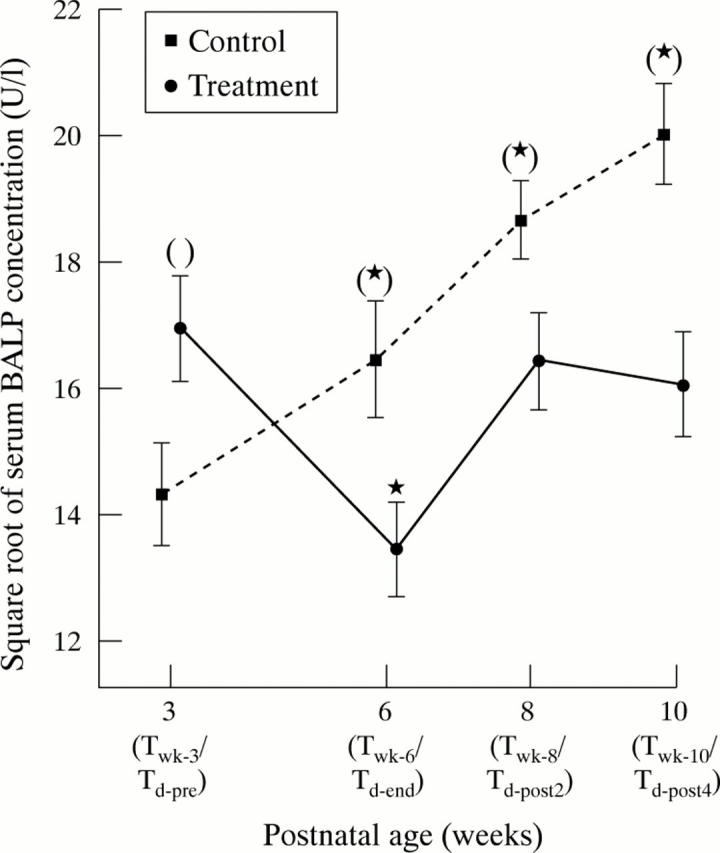
Serum bone specific alkaline phosphatase (BALP) concentrations (U/l; square root) in very low birthweight infants receiving corticosteroid or not over the study period. The asterisks indicate significant increase or decrease in serum BALP concentration compared with the pretreatment (Td-pre) or week 3 (Twk-3) levels within the same group. The parentheses indicate a significant difference in serum BALP concentration between the two groups when the corresponding time periods are compared. Results are expressed as mean and SEM.
Figure 2 .

Serum osteocalcin concentrations (ng/l; loge) in very low birthweight infants receiving corticosteroid or not over the study period. The asterisks indicate significant increase or decrease in serum osteocalcin concentration compared with the pretreatment (Td-pre) or week 3 (Twk-3) levels within the same group. The parentheses indicate a significant difference in serum osteocalcin concentration between the two groups when the corresponding time periods are compared. Results are expressed as mean and SEM.
Figure 3 .

Creatinine adjusted urinary deoxypyridinoline (Dpd) concentrations (nmol/mmol Cr) in very low birthweight infants receiving corticosteroid or not over the study period. The asterisks indicate a significant increase or decrease in creatinine adjusted urinary Dpd concentration compared with the pretreatment (Td-pre) or week 3 (Twk-3) levels within the same group. The parentheses indicate a significant difference between the two groups when the corresponding time periods are compared. Results are expressed as mean and SEM.
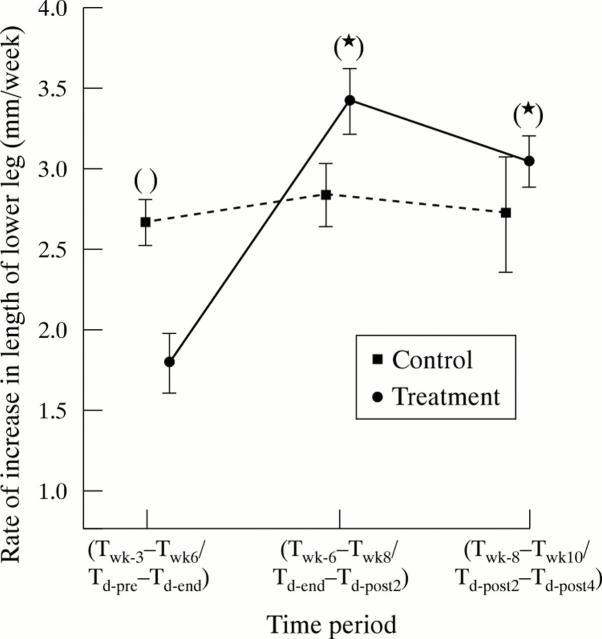
Figure 4 .
Rate of increase in the length of the lower leg (mm/week) in very low birthweight infants receiving corticosteroid or not over the study period. The asterisks indicate a significant increase or decrease in growth of the lower leg compared with the treatment period (Td-pre–Td-end) or week 3–6 of postnatal age (Twk-3–Twk-6) within the same group. The parentheses indicate a significant difference between the two groups when the corresponding time periods are compared. Results are expressed as mean and SEM.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Advani S., LaFrancis D., Bogdanovic E., Taxel P., Raisz L. G., Kream B. E. Dexamethasone suppresses in vivo levels of bone collagen synthesis in neonatal mice. Bone. 1997 Jan;20(1):41–46. doi: 10.1016/s8756-3282(96)00314-6. [DOI] [PubMed] [Google Scholar]
- Akesson K. Biochemical markers of bone turnover. A review. Acta Orthop Scand. 1995 Aug;66(4):376–386. doi: 10.3109/17453679508995567. [DOI] [PubMed] [Google Scholar]
- Altman A., Hochberg Z., Silbermann M. Interactions between growth hormone and dexamethasone in skeletal growth and bone structure of the young mouse. Calcif Tissue Int. 1992 Oct;51(4):298–304. doi: 10.1007/BF00334491. [DOI] [PubMed] [Google Scholar]
- Behr W., Barnert J. Quantification of bone alkaline phosphatase in serum by precipitation with wheat-germ lectin: a simplified method and its clinical plausibility. Clin Chem. 1986 Oct;32(10):1960–1966. [PubMed] [Google Scholar]
- Beyers N., Alheit B., Taljaard J. F., Hall J. M., Hough S. F. High turnover osteopenia in preterm babies. Bone. 1994 Jan-Feb;15(1):5–13. doi: 10.1016/8756-3282(94)90884-2. [DOI] [PubMed] [Google Scholar]
- Bhandari V., Fall P., Raisz L., Rowe J. Potential biochemical growth markers in premature infants. Am J Perinatol. 1999;16(7):339–349. doi: 10.1055/s-2007-993882. [DOI] [PubMed] [Google Scholar]
- Crofton P. M., Shrivastava A., Wade J. C., Stephen R., Kelnar CJH, Mcintosh N., Lyon A. J. Effects of dexamethasone treatment on bone and collagen turnover in preterm infants with chronic lung disease. Pediatr Res. 2000 Aug;48(2):155–162. doi: 10.1203/00006450-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Crofton P. M., Shrivastava A., Wade J. C., Stephen R., Kelnar C. J., Lyon A. J., McIntosh N. Bone and collagen markers in preterm infants: relationship with growth and bone mineral content over the first 10 weeks of life. Pediatr Res. 1999 Nov;46(5):581–587. doi: 10.1203/00006450-199911000-00015. [DOI] [PubMed] [Google Scholar]
- Doherty W. J., DeRome M. E., McCarthy M. B., Gronowicz G. A. The effect of glucocorticoids on osteoblast function. The effect of corticosterone on osteoblast expression of beta 1 integrins. J Bone Joint Surg Am. 1995 Mar;77(3):396–404. doi: 10.2106/00004623-199503000-00009. [DOI] [PubMed] [Google Scholar]
- Gibson A. T., Pearse R. G., Wales J. K. Knemometry and the assessment of growth in premature babies. Arch Dis Child. 1993 Nov;69(5 Spec No):498–504. doi: 10.1136/adc.69.5_spec_no.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer F. R. Osteopenia of prematurity. Annu Rev Nutr. 1994;14:169–185. doi: 10.1146/annurev.nu.14.070194.001125. [DOI] [PubMed] [Google Scholar]
- Gronowicz G. A., DeRome M. E., McCarthy M. B. Glucocorticoids inhibit fibronectin synthesis and messenger ribonucleic acid levels in cultured fetal rat parietal bones. Endocrinology. 1991 Feb;128(2):1107–1114. doi: 10.1210/endo-128-2-1107. [DOI] [PubMed] [Google Scholar]
- Gronowicz G. A., McCarthy M. B. Glucocorticoids inhibit the attachment of osteoblasts to bone extracellular matrix proteins and decrease beta 1-integrin levels. Endocrinology. 1995 Feb;136(2):598–608. doi: 10.1210/endo.136.2.7530648. [DOI] [PubMed] [Google Scholar]
- Kasperk C., Schneider U., Sommer U., Niethard F., Ziegler R. Differential effects of glucocorticoids on human osteoblastic cell metabolism in vitro. Calcif Tissue Int. 1995 Aug;57(2):120–126. doi: 10.1007/BF00298432. [DOI] [PubMed] [Google Scholar]
- Koo W. W., Walters J., Bush A. J., Chesney R. W., Carlson S. E. Dual-energy X-ray absorptiometry studies of bone mineral status in newborn infants. J Bone Miner Res. 1996 Jul;11(7):997–102. doi: 10.1002/jbmr.5650110717. [DOI] [PubMed] [Google Scholar]
- Lems W. F., Gerrits M. I., Jacobs J. W., van Vugt R. M., van Rijn H. J., Bijlsma J. W. Changes in (markers of) bone metabolism during high dose corticosteroid pulse treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 1996 May;55(5):288–293. doi: 10.1136/ard.55.5.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lems W. F., Jacobs J. W., van den Brink H. R., van Rijn H. J., Bijlsma J. W. Transient decrease in osteocalcin and markers of type 1 collagen turnover during high-dose corticosteroid pulse therapy in rheumatoid arthritis. Br J Rheumatol. 1993 Sep;32(9):787–789. doi: 10.1093/rheumatology/32.9.787. [DOI] [PubMed] [Google Scholar]
- Naylor K. E., Eastell R., Shattuck K. E., Alfrey A. C., Klein G. L. Bone turnover in preterm infants. Pediatr Res. 1999 Mar;45(3):363–366. doi: 10.1203/00006450-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Ng P. C. The effectiveness and side effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Arch Dis Child. 1993 Mar;68(3 Spec No):330–336. doi: 10.1136/adc.68.3_spec_no.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. K., Charles P., Mosekilde L. The effect of single oral doses of prednisone on the circadian rhythm of serum osteocalcin in normal subjects. J Clin Endocrinol Metab. 1988 Nov;67(5):1025–1030. doi: 10.1210/jcem-67-5-1025. [DOI] [PubMed] [Google Scholar]
- Tsukahara H., Sudo M., Umezaki M., Fujii Y., Kuriyama M., Yamamoto K., Ishii Y. Measurement of lumbar spinal bone mineral density in preterm infants by dual-energy X-ray absorptiometry. Biol Neonate. 1993;64(2-3):96–103. doi: 10.1159/000243978. [DOI] [PubMed] [Google Scholar]
- Tsukahara H., Takeuchi M., Fujisawa K., Miura M., Hata K., Yamamoto K., Mayumi M. High-turnover osteopenia in preterm infants: determination of urinary pyridinium cross-links of collagen. Metabolism. 1998 Mar;47(3):333–335. doi: 10.1016/s0026-0495(98)90266-9. [DOI] [PubMed] [Google Scholar]
- Ward W. E., Atkinson S. A., Donovan S. M., Paes B. Bone metabolism and circulating IGF-I and IGFBPs in dexamethasone-treated preterm infants. Early Hum Dev. 1999 Dec;56(2-3):127–141. doi: 10.1016/s0378-3782(99)00039-0. [DOI] [PubMed] [Google Scholar]