Abstract
Background: Peripheral fractional oxygen extraction (FOE) may be a better indicator of the need for transfusion than the haemoglobin concentration (Hb) because it is a measure of the adequacy of oxygen delivery to meet demand. A randomised controlled trial of the use of peripheral FOE to guide the need for blood transfusions in preterm infants was carried out to test this hypothesis.
Method: Infants less than 1500 g birth weight who were stable and less than 2 weeks old were randomised to receive transfusions guided by either a conventional protocol based on Hb (conventional group) or a protocol based on measurements of peripheral FOE made by near infrared spectroscopy (NIRS group). Measurements of Hb and FOE were made on all infants from randomisation until discharge. The primary outcome measures were number of transfusions received, rate of weight gain, and postmenstrual age at discharge.
Results: Thirty seven infants were randomised to each group. Birth weight (median, range) (1200, 1004–1373 v 1136, 1009–1285 g) and Hb (median, range) at randomisation (160, 149–179 v 155, 145–181 g/l) did not differ between the two groups. The total number of transfusions given to the NIRS group was 56 and to the conventional group 84. The median number of transfusions per infant, the median volume of blood transfused to each group, and the total number of donors to which infants were exposed were similar in the two groups. Infants transfused according to the conventional protocol were more likely to be transfused earlier and at a higher Hb than those transfused in the NIRS group. Infants in the conventional group spent a significantly shorter period than those in the NIRS group with Hb < 100 g/l. Of the 56 transfusions given to the NIRS group, 33 (59%) were given because of clinical concerns rather than because of high FOE. There was no difference in the rate of weight gain, rate of linear growth, postmenstrual age at discharge, or the incidence of chronic lung disease or retinopathy of prematurity.
Conclusions: FOE measurements failed to identify many infants felt by clinicians to require blood transfusion. This may have been because clinicians relied on conventional indicators of transfusion that are vague and non-specific, or a peripheral FOE of 0.47 alone may not be a sensitive enough predictor of the need for transfusion. This requires further study.
Full Text
The Full Text of this article is available as a PDF (115.1 KB).
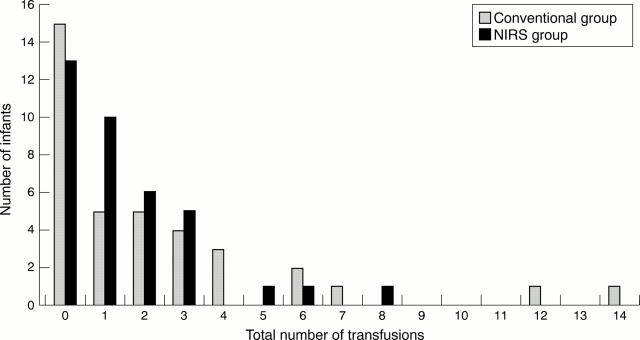
Figure 1 .
Distribution of number of transfusions per infant in the two randomised groups. NIRS, Near infrared spectroscopy.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alverson D. C., Isken V. H., Cohen R. S. Effect of booster blood transfusions on oxygen utilization in infants with bronchopulmonary dysplasia. J Pediatr. 1988 Oct;113(4):722–726. doi: 10.1016/s0022-3476(88)80389-5. [DOI] [PubMed] [Google Scholar]
- Alverson D. C. The physiologic impact of anemia in the neonate. Clin Perinatol. 1995 Sep;22(3):609–625. [PubMed] [Google Scholar]
- Blank J. P., Sheagren T. G., Vajaria J., Mangurten H. H., Benawra R. S., Puppala B. L. The role of RBC transfusion in the premature infant. Am J Dis Child. 1984 Sep;138(9):831–833. doi: 10.1001/archpedi.1984.02140470031010. [DOI] [PubMed] [Google Scholar]
- Cochran D. P., Shaw N. J. The use of pulse oximetry in the prevention of hyperoxaemia in preterm infants. Eur J Pediatr. 1995 Mar;154(3):222–224. doi: 10.1007/BF01954276. [DOI] [PubMed] [Google Scholar]
- Cooke R. W., Clark D., Hickey-Dwyer M., Weindling A. M. The apparent role of blood transfusions in the development of retinopathy of prematurity. Eur J Pediatr. 1993 Oct;152(10):833–836. doi: 10.1007/BF02073381. [DOI] [PubMed] [Google Scholar]
- Holland B. M., Jones J. G., Wardrop C. A. Lessons from the anemia of prematurity. Hematol Oncol Clin North Am. 1987 Sep;1(3):355–366. [PubMed] [Google Scholar]
- Jones J. G., Holland B. M., Hudson I. R., Wardrop C. A. Total circulating red cells versus haematocrit as the primary descriptor of oxygen transport by the blood. Br J Haematol. 1990 Oct;76(2):288–294. doi: 10.1111/j.1365-2141.1990.tb07886.x. [DOI] [PubMed] [Google Scholar]
- Jones J. G., Holland B. M., Veale K. E., Wardrop C. A. 'Available oxygen', a realistic expression of the ability of the blood to supply oxygen to tissues. Scand J Haematol. 1979 Jan;22(1):77–82. doi: 10.1111/j.1600-0609.1979.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Lachance C., Chessex P., Fouron J. C., Widness J. A., Bard H. Myocardial, erythropoietic, and metabolic adaptations to anemia of prematurity. J Pediatr. 1994 Aug;125(2):278–282. doi: 10.1016/s0022-3476(94)70211-x. [DOI] [PubMed] [Google Scholar]
- Lister G., Moreau G., Moss M., Talner N. S. Effects of alterations of oxygen transport on the neonate. Semin Perinatol. 1984 Jul;8(3):192–204. [PubMed] [Google Scholar]
- Meyer J., Sive A., Jacobs P. Empiric red cell transfusion in asymptomatic preterm infants. Acta Paediatr. 1993 Jan;82(1):30–34. doi: 10.1111/j.1651-2227.1993.tb12510.x. [DOI] [PubMed] [Google Scholar]
- Sacks L. M., Schaffer D. B., Anday E. K., Peckham G. J., Delivoria-Papadopoulos M. Retrolental fibroplasia and blood transfusion in very low-birth-weight infants. Pediatrics. 1981 Dec;68(6):770–774. [PubMed] [Google Scholar]
- Shannon K. M., Keith J. F., 3rd, Mentzer W. C., Ehrenkranz R. A., Brown M. S., Widness J. A., Gleason C. A., Bifano E. M., Millard D. D., Davis C. B. Recombinant human erythropoietin stimulates erythropoiesis and reduces erythrocyte transfusions in very low birth weight preterm infants. Pediatrics. 1995 Jan;95(1):1–8. [PubMed] [Google Scholar]
- Shohat M., Reisner S. H., Krikler R., Nissenkorn I., Yassur Y., Ben-Sira I. Retinopathy of prematurity: incidence and risk factors. Pediatrics. 1983 Aug;72(2):159–163. [PubMed] [Google Scholar]
- Stockman J. A., 3rd, Clark D. A. Weight gain: a response to transfusion in selected preterm infants. Am J Dis Child. 1984 Sep;138(9):828–830. doi: 10.1001/archpedi.1984.02140470028009. [DOI] [PubMed] [Google Scholar]
- Strauss R. G. Red blood cell transfusion practices in the neonate. Clin Perinatol. 1995 Sep;22(3):641–655. [PubMed] [Google Scholar]
- Sullivan J. L. Iron, plasma antioxidants, and the 'oxygen radical disease of prematurity'. Am J Dis Child. 1988 Dec;142(12):1341–1344. doi: 10.1001/archpedi.1988.02150120095048. [DOI] [PubMed] [Google Scholar]
- Wardle S. P., Yoxall C. W., Crawley E., Weindling A. M. Peripheral oxygenation and anemia in preterm babies. Pediatr Res. 1998 Jul;44(1):125–131. doi: 10.1203/00006450-199807000-00020. [DOI] [PubMed] [Google Scholar]
- Wardrop C. A., Holland B. M., Veale K. E., Jones J. G., Gray O. P. Nonphysiological anaemia of prematurity. Arch Dis Child. 1978 Nov;53(11):855–860. doi: 10.1136/adc.53.11.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoxall C. W., Weindling A. M. Measurement of venous oxyhaemoglobin saturation in the adult human forearm by near infrared spectroscopy with venous occlusion. Med Biol Eng Comput. 1997 Jul;35(4):331–336. doi: 10.1007/BF02534086. [DOI] [PubMed] [Google Scholar]
- Yoxall C. W., Weindling A. M. The measurement of peripheral venous oxyhemoglobin saturation in newborn infants by near infrared spectroscopy with venous occlusion. Pediatr Res. 1996 Jun;39(6):1103–1106. doi: 10.1203/00006450-199606000-00028. [DOI] [PubMed] [Google Scholar]