Full Text
The Full Text of this article is available as a PDF (98.1 KB).
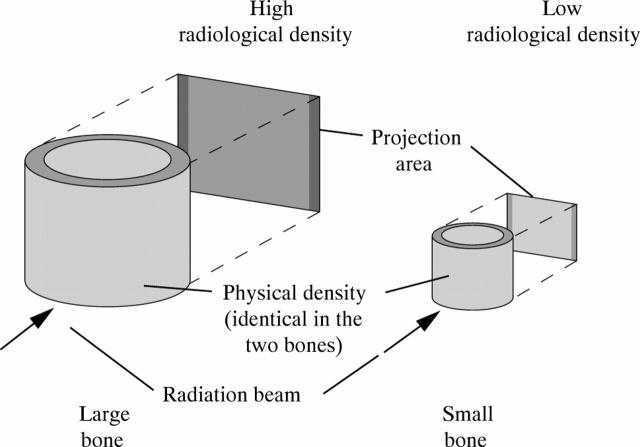
Figure 1 .
Comparison of density, as defined in physics, and radiological (areal) bone density, as commonly used in the clinical setting. The two bones have the same physical density, but the larger bone appears denser when projected on to a screen. The reason is that it absorbs more radiation because of the longer path length of the radiation beam through the bone. This is analogous to comparing the shadows of two differently sized bottles placed in the sun. The larger bottle will have a darker shadow, even if the two bottles are made of the same material and contain the same liquid. Similarly, the areal bone density of a premature infant with a birth weight of 1000 g is lower than that of a healthy term newborn, even if the physical density of the bones is identical.
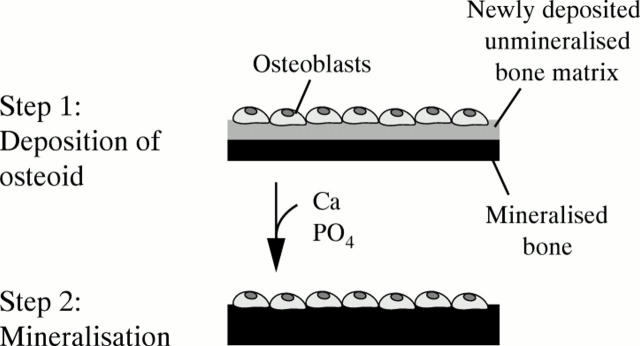
Figure 2 .
Schematic representation of the bone formation process. Firstly, a team of osteoblasts synthesises organic bone matrix and deposits it on an already existing surface of mineralised bone. This can be the surface of a trabecula or of the bone's cortex. In a second step occurring several days later, mineral is added to the newly deposited organic matrix.
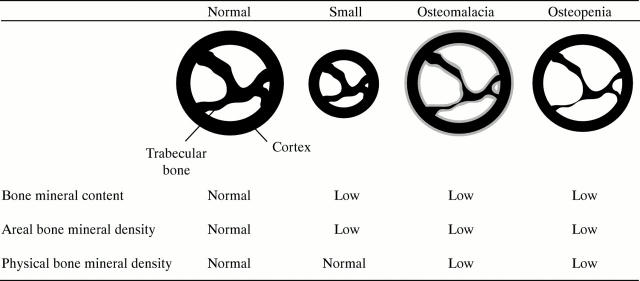
Figure 3 .
Conditions affecting bone mineral content and density in premature infants. Shown are schematic bone cross sections. Mineralised bone matrix is indicated in black, and unmineralised bone matrix in grey. The small bone has a lower bone mineral content and areal bone mineral density than the normally sized bone, although it is otherwise completely normal. In osteomalacia, there is too much unmineralised bone matrix because not enough mineral has been incorporated into the matrix (mineralisation defect). In osteopenia, there is not enough bone matrix, but the matrix that is there has been filled with mineral in a normal fashion (no mineralisation defect).
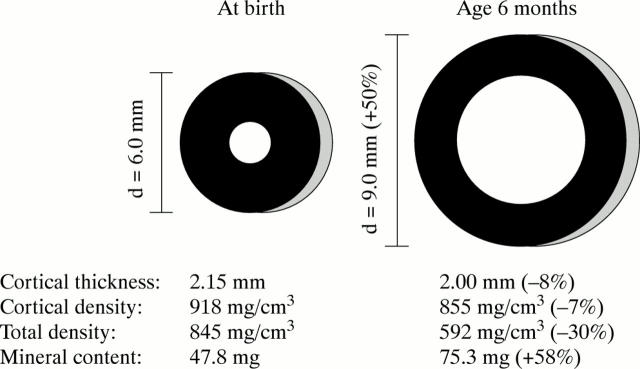
Figure 4 .
Normal postnatal changes in the cross section of the femoral diaphysis. During the first six months of life, external bone size (bone diameter, d) increases by about 50%, whereas the thickness of the bone cortex decreases slightly. The total physical density of the femoral shaft—that is, density averaged over the whole cross section—decreases by about 30%, although the physical density of the cortex—that is, density averaged over the compartment shown in black—decreases only by 7%. The difference is due to the relatively larger marrow cavity which does not contain bone mineral. Even though total physical density decreases, the absolute amount of mineral increases (in this example total mineral content is calculated for a hypothetical 2 mm thick slice of the femur). However, the functionally most important aspect of these developments is that bone strength increases about threefold because of the changes in bone geometry.11
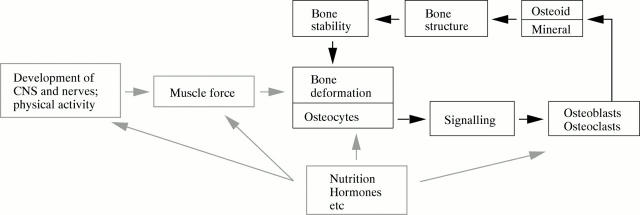
Figure 5 .
The mechanostat model of bone development according to Frost.25, 26 The central regulatory unit of bone development is the feedback mechanism between bone deformation and bone stability (black boxes and thick black arrows). Osteocytes act as sensors of bone deformation. If deformation exceeds a preset threshold, osteocytes cause osteoblasts and osteoclasts to adapt the bone tissue to the increased load. Thereby bone stability increases and the bone is deformed less by the same load. In children, the loads on the bones increase continuously as the result of growth in length and increasing muscle force. Consequently, bone stability is continuously adapted "upwards". However, during inactivity, bone stability can also be adapted "downwards"—that is, the bones get weaker. Nutrition, hormones, and other factors modulate the regulatory feedback loop at various sites (grey boxes and arrows).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backström M. C., Kuusela A. L., Mäki R. Metabolic bone disease of prematurity. Ann Med. 1996 Aug;28(4):275–282. doi: 10.3109/07853899608999080. [DOI] [PubMed] [Google Scholar]
- Bishop N. Bone disease in preterm infants. Arch Dis Child. 1989 Oct;64(10 Spec No):1403–1409. doi: 10.1136/adc.64.10_spec_no.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon P. J., Horsman A., Ryan S. W., Truscott J. G., Durward H. Spontaneous resolution of bone mineral depletion in preterm infants. Arch Dis Child. 1990 Oct;65(10 Spec No):1038–1042. doi: 10.1136/adc.65.10_spec_no.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlenburg S. L., Bishop N. J., Lucas A. Are preterm infants at risk for subsequent fractures? Arch Dis Child. 1989 Oct;64(10 Spec No):1384–1385. doi: 10.1136/adc.64.10_spec_no.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewtrell M. S., Prentice A., Jones S. C., Bishop N. J., Stirling D., Buffenstein R., Lunt M., Cole T. J., Lucas A. Bone mineralization and turnover in preterm infants at 8-12 years of age: the effect of early diet. J Bone Miner Res. 1999 May;14(5):810–820. doi: 10.1359/jbmr.1999.14.5.810. [DOI] [PubMed] [Google Scholar]
- Frost H. M., Schönau E. The "muscle-bone unit" in children and adolescents: a 2000 overview. J Pediatr Endocrinol Metab. 2000 Jun;13(6):571–590. doi: 10.1515/jpem.2000.13.6.571. [DOI] [PubMed] [Google Scholar]
- Greer F. R. Osteopenia of prematurity. Annu Rev Nutr. 1994;14:169–185. doi: 10.1146/annurev.nu.14.070194.001125. [DOI] [PubMed] [Google Scholar]
- Helin I., Landin L. A., Nilsson B. E. Bone mineral content in preterm infants at age 4 to 16. Acta Paediatr Scand. 1985 Mar;74(2):264–267. doi: 10.1111/j.1651-2227.1985.tb10962.x. [DOI] [PubMed] [Google Scholar]
- Hori C., Tsukahara H., Fujii Y., Kawamitsu T., Konishi Y., Yamamoto K., Ishii Y., Sudo M. Bone mineral status in preterm-born children: assessment by dual-energy X-ray absorptiometry. Biol Neonate. 1995;68(4):254–258. doi: 10.1159/000244243. [DOI] [PubMed] [Google Scholar]
- Horsman A., Ryan S. W., Congdon P. J., Truscott J. G., Simpson M. Bone mineral content and body size 65 to 100 weeks' postconception in preterm and full term infants. Arch Dis Child. 1989 Nov;64(11):1579–1586. doi: 10.1136/adc.64.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J. A., Melton L. J., 3rd, Christiansen C., Johnston C. C., Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994 Aug;9(8):1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- Lapillonne A. A., Glorieux F. H., Salle B. L., Braillon P. M., Chambon M., Rigo J., Putet G., Senterre J. Mineral balance and whole body bone mineral content in very low-birth-weight infants. Acta Paediatr Suppl. 1994 Dec;405:117–122. doi: 10.1111/j.1651-2227.1994.tb13409.x. [DOI] [PubMed] [Google Scholar]
- Moyer-Mileur L. J., Brunstetter V., McNaught T. P., Gill G., Chan G. M. Daily physical activity program increases bone mineralization and growth in preterm very low birth weight infants. Pediatrics. 2000 Nov;106(5):1088–1092. doi: 10.1542/peds.106.5.1088. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Koo W. W. Interpretation of absorptiometric bone mass measurements in the growing skeleton: issues and limitations. Calcif Tissue Int. 1999 Jul;65(1):1–3. doi: 10.1007/s002239900648. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987 Dec;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Rauch F., Schoenau E. Changes in bone density during childhood and adolescence: an approach based on bone's biological organization. J Bone Miner Res. 2001 Apr;16(4):597–604. doi: 10.1359/jbmr.2001.16.4.597. [DOI] [PubMed] [Google Scholar]
- Rauch F., Schoenau E. The developing bone: slave or master of its cells and molecules? Pediatr Res. 2001 Sep;50(3):309–314. doi: 10.1203/00006450-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Rigo J., De Curtis M., Pieltain C., Picaud J. C., Salle B. L., Senterre J. Bone mineral metabolism in the micropremie. Clin Perinatol. 2000 Mar;27(1):147–170. doi: 10.1016/s0095-5108(05)70011-7. [DOI] [PubMed] [Google Scholar]
- Rodríguez J. I., Garcia-Alix A., Palacios J., Paniagua R. Changes in the long bones due to fetal immobility caused by neuromuscular disease. A radiographic and histological study. J Bone Joint Surg Am. 1988 Aug;70(7):1052–1060. [PubMed] [Google Scholar]
- Rodríguez J. I., Palacios J., García-Alix A., Pastor I., Paniagua R. Effects of immobilization on fetal bone development. A morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif Tissue Int. 1988 Dec;43(6):335–339. doi: 10.1007/BF02553275. [DOI] [PubMed] [Google Scholar]
- Schanler R. J., Burns P. A., Abrams S. A., Garza C. Bone mineralization outcomes in human milk-fed preterm infants. Pediatr Res. 1992 Jun;31(6):583–586. doi: 10.1203/00006450-199206000-00009. [DOI] [PubMed] [Google Scholar]