Abstract
Objectives: To report 18 month outcome of a randomised trial of two courses of dexamethasone to prevent chronic lung disease of prematurity.
Study design: Babies of birth weight 1250 g or less ventilated at 7 days of age were randomised to a 42 day reducing course (long) or a 3 day pulsed (pulse) course of dexamethasone.
Growth, cardiovascular status, and respiratory and neurodevelopmental outcomes were assessed at 18 months.
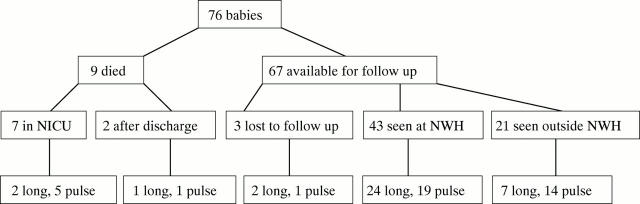
Results: Seventy six babies were enrolled. Nine died and three were lost to follow up. Babies receiving the long course were weaned off oxygen more quickly than those receiving the pulse course (47% v 69% on oxygen at 28 days; p = 0.01), but there were no differences in 18 month outcomes. However, children averaged -1 SD for growth parameters, half had moderate or severe disability, and 35% and 19% respectively required oxygen at 36 weeks and discharge.
Conclusions: The dexamethasone course used did not influence long term outcome. However, entry criteria for this study selected a group of babies at high risk of poor long term outcome.
Full Text
The Full Text of this article is available as a PDF (154.7 KB).
Figure 1 .
Follow up details of babies enrolled in each study group: long, 42 day reducing course of dexamethasone; pulse, three day pulsed course of dexamethasone. NICU, Neonatal intensive care unit; NWH, National Women's Hospital.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensky A. S., Kothadia J. M., Covitz W. Cardiac effects of dexamethasone in very low birth weight infants. Pediatrics. 1996 Jun;97(6 Pt 1):818–821. [PubMed] [Google Scholar]
- Bhuta T., Ohlsson A. Systematic review and meta-analysis of early postnatal dexamethasone for prevention of chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 1998 Jul;79(1):F26–F33. doi: 10.1136/fn.79.1.f26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield F. H., Knight D. B., Harding J. E. Side effects of 2 different dexamethasone courses for preterm infants at risk of chronic lung disease: a randomized trial. J Pediatr. 1998 Sep;133(3):395–400. doi: 10.1016/s0022-3476(98)70277-x. [DOI] [PubMed] [Google Scholar]
- Breitweser J. A., Meyer R. A., Sperling M. A., Tsang R. C., Kaplan S. Cardiac septal hypertrophy in hyperinsulinemic infants. J Pediatr. 1980 Mar;96(3 Pt 2):535–539. doi: 10.1016/s0022-3476(80)80862-6. [DOI] [PubMed] [Google Scholar]
- Brozanski B. S., Jones J. G., Gilmour C. H., Balsan M. J., Vazquez R. L., Israel B. A., Newman B., Mimouni F. B., Guthrie R. D. Effect of pulse dexamethasone therapy on the incidence and severity of chronic lung disease in the very low birth weight infant. J Pediatr. 1995 May;126(5 Pt 1):769–776. doi: 10.1016/s0022-3476(95)70410-8. [DOI] [PubMed] [Google Scholar]
- Crofton P. M., Stirling H. F., Schönau E., Ahmed S. F., Wallace W. H., Wade J. C., Magowan R., Shrivastava A., Lyon A. J., McIntosh N. Biochemical markers of bone turnover. Horm Res. 1996;45 (Suppl 1):55–58. doi: 10.1159/000184832. [DOI] [PubMed] [Google Scholar]
- Cummings J. J., D'Eugenio D. B., Gross S. J. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med. 1989 Jun 8;320(23):1505–1510. doi: 10.1056/NEJM198906083202301. [DOI] [PubMed] [Google Scholar]
- Durand M., Sardesai S., McEvoy C. Effects of early dexamethasone therapy on pulmonary mechanics and chronic lung disease in very low birth weight infants: a randomized, controlled trial. Pediatrics. 1995 Apr;95(4):584–590. [PubMed] [Google Scholar]
- Groneck P., Oppermann M., Speer C. P. Levels of complement anaphylatoxin C5a in pulmonary effluent fluid of infants at risk for chronic lung disease and effects of dexamethasone treatment. Pediatr Res. 1993 Nov;34(5):586–590. doi: 10.1203/00006450-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Harkavy K. L., Scanlon J. W., Chowdhry P. K., Grylack L. J. Dexamethasone therapy for chronic lung disease in ventilator- and oxygen-dependent infants: a controlled trial. J Pediatr. 1989 Dec;115(6):979–983. doi: 10.1016/s0022-3476(89)80754-1. [DOI] [PubMed] [Google Scholar]
- Howard E., Benjamins J. A. DNA, ganglioside and sulfatide in brains of rats given corticosterone in infancy, with an estimate of cell loss during development. Brain Res. 1975 Jul 4;92(1):73–87. doi: 10.1016/0006-8993(75)90528-4. [DOI] [PubMed] [Google Scholar]
- Kitchen W. H., Ford G. W., Rickards A. L., Lissenden J. V., Ryan M. M. Children of birth weight less than 1000 g: changing outcome between ages 2 and 5 years. J Pediatr. 1987 Feb;110(2):283–288. doi: 10.1016/s0022-3476(87)80174-9. [DOI] [PubMed] [Google Scholar]
- Marinelli K. A., Burke G. S., Herson V. C. Effects of dexamethasone on blood pressure in premature infants with bronchopulmonary dysplasia. J Pediatr. 1997 Apr;130(4):594–602. doi: 10.1016/s0022-3476(97)70244-0. [DOI] [PubMed] [Google Scholar]
- Meisels S. J., Plunkett J. W., Roloff D. W., Pasick P. L., Stiefel G. S. Growth and development of preterm infants with respiratory distress syndrome and bronchopulmonary dysplasia. Pediatrics. 1986 Mar;77(3):345–352. [PubMed] [Google Scholar]
- Merritt T. A., Stuard I. D., Puccia J., Wood B., Edwards D. K., Finkelstein J., Shapiro D. L. Newborn tracheal aspirate cytology: classification during respiratory distress syndrome and bronchopulmonary dysplasia. J Pediatr. 1981 Jun;98(6):949–956. doi: 10.1016/s0022-3476(81)80603-8. [DOI] [PubMed] [Google Scholar]
- Michaelsen K. F., Skov L., Badsberg J. H., Jørgensen M. Short-term measurement of linear growth in preterm infants: validation of a hand-held knemometer. Pediatr Res. 1991 Nov;30(5):464–468. doi: 10.1203/00006450-199111000-00013. [DOI] [PubMed] [Google Scholar]
- O'Shea T. M., Kothadia J. M., Klinepeter K. L., Goldstein D. J., Jackson B. G., Weaver R. G., 3rd, Dillard R. G. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics. 1999 Jul;104(1 Pt 1):15–21. doi: 10.1542/peds.104.1.15. [DOI] [PubMed] [Google Scholar]
- Papile L. A., Tyson J. E., Stoll B. J., Wright L. L., Donovan E. F., Bauer C. R., Krause-Steinrauf H., Verter J., Korones S. B., Lemons J. A. A multicenter trial of two dexamethasone regimens in ventilator-dependent premature infants. N Engl J Med. 1998 Apr 16;338(16):1112–1118. doi: 10.1056/NEJM199804163381604. [DOI] [PubMed] [Google Scholar]
- Rastogi A., Akintorin S. M., Bez M. L., Morales P., Pildes R. S. A controlled trial of dexamethasone to prevent bronchopulmonary dysplasia in surfactant-treated infants. Pediatrics. 1996 Aug;98(2 Pt 1):204–210. [PubMed] [Google Scholar]
- Robertson C. M., Etches P. C., Goldson E., Kyle J. M. Eight-year school performance, neurodevelopmental, and growth outcome of neonates with bronchopulmonary dysplasia: a comparative study. Pediatrics. 1992 Mar;89(3):365–372. [PubMed] [Google Scholar]
- Rogé C. L., Silverman N. H., Hart P. A., Ray R. M. Cardiac structure growth pattern determined by echocardiography. Circulation. 1978 Feb;57(2):285–290. doi: 10.1161/01.cir.57.2.285. [DOI] [PubMed] [Google Scholar]
- Skelton R., Gill A. B., Parsons J. M. Cardiac effects of short course dexamethasone in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998 Mar;78(2):F133–F137. doi: 10.1136/fn.78.2.f133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore M. D., Rivers A., Hack M. Increased risk of cerebral palsy among very low-birthweight infants with chronic lung disease. Dev Med Child Neurol. 1990 Apr;32(4):325–332. doi: 10.1111/j.1469-8749.1990.tb16944.x. [DOI] [PubMed] [Google Scholar]
- Tarnow-Mordi W., Mitra A. Postnatal dexamethasone in preterm infants is potentially lifesaving, but follow up studies are urgently needed. BMJ. 1999 Nov 27;319(7222):1385–1386. doi: 10.1136/bmj.319.7222.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrlenich L. A., Bozynski M. E., Shyr Y., Schork M. A., Roloff D. W., McCormick M. C. The effect of bronchopulmonary dysplasia on growth at school age. Pediatrics. 1995 Jun;95(6):855–859. [PubMed] [Google Scholar]
- Wilson D. M., Baldwin R. B., Ariagno R. L. A randomized, placebo-controlled trial of effects of dexamethasone on hypothalamic-pituitary-adrenal axis in preterm infants. J Pediatr. 1988 Oct;113(4):764–768. doi: 10.1016/s0022-3476(88)80398-6. [DOI] [PubMed] [Google Scholar]