Abstract
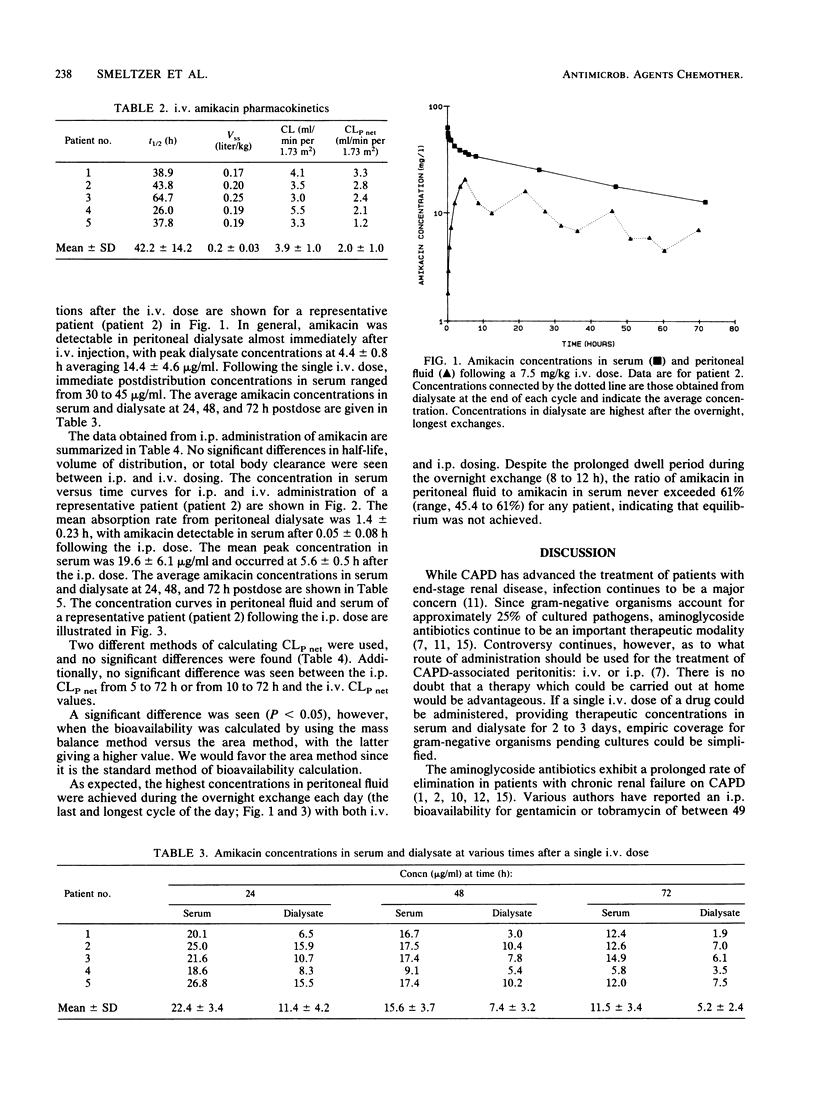
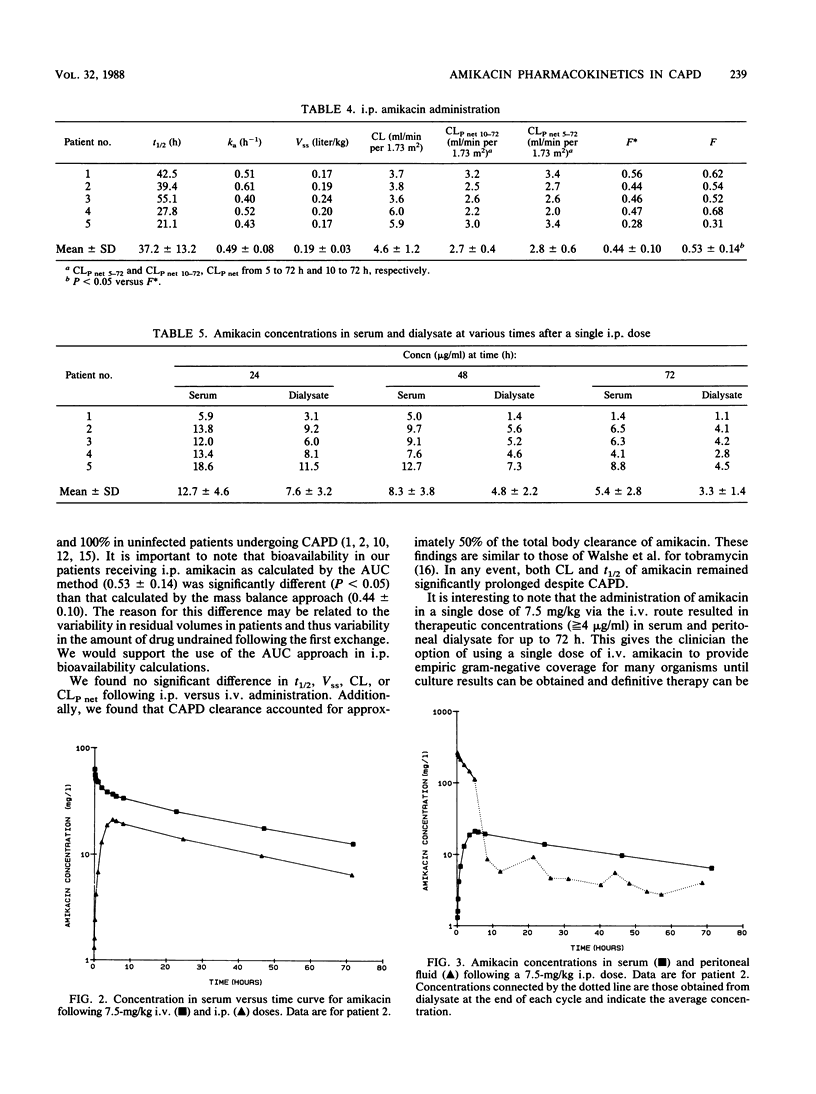
The pharmacokinetics of amikacin were investigated in five stable patients undergoing continuous ambulatory peritoneal dialysis (CAPD). Each patient was studied after the administration of 7.5 mg of amikacin per kg by both the intravenous (i.v.) and intraperitoneal (i.p.) route, allowing a 1-month washout period between doses. No differences in amikacin half-life, volume of distribution, total body clearance, or time-averaged peritoneal clearance were noted between the two routes of administration. After a 5-h dwell period, bioavailability as calculated by the area under the curve for i.p. amikacin was 53 +/- 14.0%. Amikacin pharmacokinetics parallel those of other aminoglycosides in CAPD patients when the drug is administered either i.v. or i.p. Single loading doses of amikacin administered i.v. to uninfected CAPD patients provided therapeutic serum and dialysate levels for many aerobic gram-negative organisms for up to 72 h. Because of the variability of absorption of i.p. administered amikacin, single i.p. doses are not recommended.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunke C. M., Aronoff G. R., Brier M. E., Sloan R. S., Luft F. C. Tobramycin kinetics during continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther. 1983 Jul;34(1):110–116. doi: 10.1038/clpt.1983.138. [DOI] [PubMed] [Google Scholar]
- Janicke D. M., Morse G. D., Apicella M. A., Jusko W. J., Walshe J. J. Pharmacokinetic modeling of bidirectional transfer during peritoneal dialysis. Clin Pharmacol Ther. 1986 Aug;40(2):209–218. doi: 10.1038/clpt.1986.165. [DOI] [PubMed] [Google Scholar]
- Morse G. D., Farolino D. F., Apicella M. A., Walshe J. J. Comparative study of intraperitoneal and intravenous vancomycin pharmacokinetics during continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1987 Feb;31(2):173–177. doi: 10.1128/aac.31.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreopoulos D. G., Robson M., Izatt S., Clayton S., deVeber G. A. A simple and safe technique for continuous ambulatory peritoneal dialysis (CAPD). Trans Am Soc Artif Intern Organs. 1978;24:484–489. [PubMed] [Google Scholar]
- Pancorbo S., Comty C. Pharmacokinetics of gentamicin in patients undergoing continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1981 Apr;19(4):605–607. doi: 10.1128/aac.19.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Matzke G., Keane W. F. Current concepts in the management of peritonitis in patients undergoing continuous ambulatory peritoneal dialysis. Rev Infect Dis. 1987 May-Jun;9(3):604–612. doi: 10.1093/clinids/9.3.604. [DOI] [PubMed] [Google Scholar]
- Ruckdeschel J. C., Schimpff S. C., Smyth A. C., Mardiney M. R., Jr Herpes zoster and impaired cell-associated immunity to the varicella-zoster virus in patients with Hodgkin's disease. Am J Med. 1977 Jan;62(1):77–85. doi: 10.1016/0002-9343(77)90352-7. [DOI] [PubMed] [Google Scholar]
- Somani P., Shapiro R. S., Stockard H., Higgins J. T. Unidirectional absorption of gentamicin from the peritoneum during continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther. 1982 Jul;32(1):113–121. doi: 10.1038/clpt.1982.134. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Cannon J. G., So M. Phase and antigenic variation of pili and outer membrane protein II of Neisseria gonorrhoeae. J Infect Dis. 1986 Feb;153(2):196–201. doi: 10.1093/infdis/153.2.196. [DOI] [PubMed] [Google Scholar]
- Vas S. I. Microbiologic aspects of chronic ambulatory peritoneal dialysis. Kidney Int. 1983 Jan;23(1):83–92. doi: 10.1038/ki.1983.15. [DOI] [PubMed] [Google Scholar]
- WAGNER J. G., NELSON E. KINETIC ANALYSIS OF BLOOD LEVELS AND URINARY EXCRETION IN THE ABSORPTIVE PHASE AFTER SINGLE DOSES OF DRUG. J Pharm Sci. 1964 Nov;53:1392–1403. doi: 10.1002/jps.2600531126. [DOI] [PubMed] [Google Scholar]
- de Paepe M., Lameire N., Belpaire F., Bogaert M. Peritoneal pharmacokinetics of gentamicin in man. Clin Nephrol. 1983 Mar;19(3):107–109. [PubMed] [Google Scholar]


