Abstract
Background: The pathogenesis of posthaemorrhagic hydrocephalus (PHHC) following intraventricular haemorrhage (IVH) in premature infants includes a fibroproliferative reaction leading to arachnoidal fibrosis, ultimately causing malresorption of cerebrospinal fluid (CSF) at the arachnoid villi.
Aims: To determine whether an increased concentration of the carboxyterminal propeptide of type I procollagen (PICP) in the CSF of neonates after IVH reflects the activation of collagen synthesis preceding the manifestation of PHHC.
Methods: From 20 neonates with PHHC (median birth weight 740 g, median gestational age 25+1 weeks), 52 CSF samples were collected. CSF samples of four neonates (median birth weight 2170 g, median gestational age 32+4 weeks) with congenital non-haemorrhagic hydrocephalus served as controls. PICP was measured by radioimmunoassay.
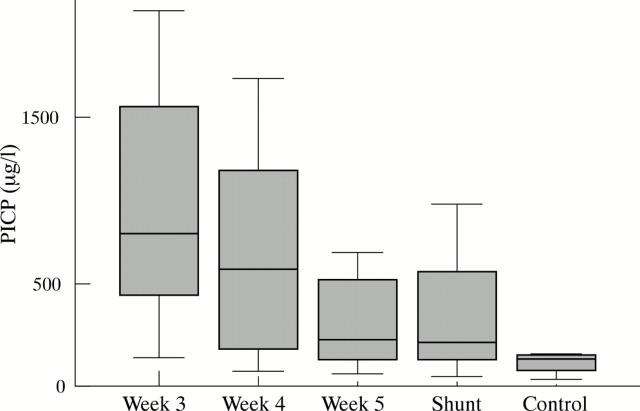
Results: PICP in CSF taken at the start of external CSF drainage (median day 21, range 17–25 days postnatal age) was significantly increased (median 851.5, range 153.5–1944 µg/l) compared with controls (median 136.1, range 33.8–169.5 µg/l). CSF concentrations of PICP declined until permanent shunt placement (median day 70, range days 41–113).
Conclusion: In neonates who develop PHHC, significant elevation of PICP concentration in the CSF is present 3–4 weeks after IVH. It reflects the increase of local type I collagen turnover, thereby correlating with manifestation of PHHC.
Full Text
The Full Text of this article is available as a PDF (77.8 KB).
Figure 1 .
Time course of PICP concentrations in the CSF of 20 neonates with PHHC and four control subjects. Values are median and 25th and 75th centiles (boxes), and 5th and 95th centiles (whiskers).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmann P. A., Lazzara A., Dykes F. D., Brann A. W., Jr, Schwartz J. F. Intraventricular hemorrhage in the high-risk preterm infant: incidence and outcome. Ann Neurol. 1980 Feb;7(2):118–124. doi: 10.1002/ana.410070205. [DOI] [PubMed] [Google Scholar]
- Dykes F. D., Dunbar B., Lazarra A., Ahmann P. A. Posthemorrhagic hydrocephalus in high-risk preterm infants: natural history, management, and long-term outcome. J Pediatr. 1989 Apr;114(4 Pt 1):611–618. doi: 10.1016/s0022-3476(89)80707-3. [DOI] [PubMed] [Google Scholar]
- Hansen A. R., Volpe J. J., Goumnerova L. C., Madsen J. R. Intraventricular urokinase for the treatment of posthemorrhagic hydrocephalus. Pediatr Neurol. 1997 Oct;17(3):213–217. doi: 10.1016/s0887-8994(97)00130-6. [DOI] [PubMed] [Google Scholar]
- Hansen A., Whitelaw A., Lapp C., Brugnara C. Cerebrospinal fluid plasminogen activator inhibitor-1: a prognostic factor in posthaemorrhagic hydrocephalus. Acta Paediatr. 1997 Sep;86(9):995–998. doi: 10.1111/j.1651-2227.1997.tb15186.x. [DOI] [PubMed] [Google Scholar]
- Heep A., Engelskirchen R., Holschneider A., Groneck P. Primary intervention for posthemorrhagic hydrocephalus in very low birthweight infants by ventriculostomy. Childs Nerv Syst. 2001 Jan;17(1-2):47–51. doi: 10.1007/s003810000363. [DOI] [PubMed] [Google Scholar]
- Kim R. C., Talbert W. M., Choe W., Choi B. H. Massive craniospinal collagen deposition after persistent postoperative intraventricular bleeding. Neurosurgery. 1989 May;24(5):771–775. doi: 10.1227/00006123-198905000-00022. [DOI] [PubMed] [Google Scholar]
- Levene M. I., Starte D. R. A longitudinal study of post-haemorrhagic ventricular dilatation in the newborn. Arch Dis Child. 1981 Dec;56(12):905–910. doi: 10.1136/adc.56.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano R., Velardi F., Romagnoli C., Papacci P., De Stefano V., Tortorolo G. Failure of fibrinolytic endoventricular treatment to prevent neonatal post-haemorrhagic hydrocephalus. A case-control trial. Childs Nerv Syst. 1997 Feb;13(2):73–76. doi: 10.1007/s003810050045. [DOI] [PubMed] [Google Scholar]
- Massicotte E. M., Del Bigio M. R. Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg. 1999 Jul;91(1):80–84. doi: 10.3171/jns.1999.91.1.0080. [DOI] [PubMed] [Google Scholar]
- Motohashi O., Suzuki M., Shida N., Umezawa K., Ohtoh T., Sakurai Y., Yoshimoto T. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemical study. Acta Neurochir (Wien) 1995;136(1-2):88–91. doi: 10.1007/BF01411441. [DOI] [PubMed] [Google Scholar]
- Pang D., Sclabassi R. J., Horton J. A. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986 Oct;19(4):553–572. doi: 10.1227/00006123-198610000-00010. [DOI] [PubMed] [Google Scholar]
- Papile L. A., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978 Apr;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- Raghow R., Lurie S., Seyer J. M., Kang A. H. Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest. 1985 Nov;76(5):1733–1739. doi: 10.1172/JCI112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Risteli L. Analysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspects. J Hepatol. 1995;22(2 Suppl):77–81. [PubMed] [Google Scholar]
- Risteli L., Risteli J. Analysis of extracellular matrix proteins in biological fluids. Methods Enzymol. 1987;145:391–411. doi: 10.1016/0076-6879(87)45022-2. [DOI] [PubMed] [Google Scholar]
- Sajanti J., Björkstrand A. S., Finnilä S., Heikkinen E., Peltonen J., Majamaa K. Increase of collagen synthesis and deposition in the arachnoid and the dura following subarachnoid hemorrhage in the rat. Biochim Biophys Acta. 1999 Aug 30;1454(3):209–216. doi: 10.1016/s0925-4439(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Sajanti J., Heikkinen E., Majamaa K. Transient increase in procollagen propeptides in the CSF after subarachnoid hemorrhage. Neurology. 2000 Aug 8;55(3):359–363. doi: 10.1212/wnl.55.3.359. [DOI] [PubMed] [Google Scholar]
- Sajanti J., Majamaa K. Detection of meningeal fibrosis after subarachnoid haemorrhage by assaying procollagen propeptides in cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1999 Aug;67(2):185–188. doi: 10.1136/jnnp.67.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold-Weiger K., Wollmann H. A., Ranke M. B., Speer C. P. Plasma concentrations of the carboxyterminal propeptide of type I procollagen (PICP) in preterm neonates from birth to term. Pediatr Res. 2000 Jul;48(1):104–108. doi: 10.1203/00006450-200007000-00018. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Ishii M., Ottomo M., Iwabuchi T. Changes in the subarachnoid space after experimental subarachnoid haemorrhage in the dog: scanning electron microscopic observation. Acta Neurochir (Wien) 1977;39(1-2):1–14. doi: 10.1007/BF01405236. [DOI] [PubMed] [Google Scholar]
- Synnes A. R., Chien L. Y., Peliowski A., Baboolal R., Lee S. K., Canadian NICU Network Variations in intraventricular hemorrhage incidence rates among Canadian neonatal intensive care units. J Pediatr. 2001 Apr;138(4):525–531. doi: 10.1067/mpd.2001.111822. [DOI] [PubMed] [Google Scholar]
- Tortorolo G., Luciano R., Papacci P., Tonelli T. Intraventricular hemorrhage: past, present and future, focusing on classification, pathogenesis and prevention. Childs Nerv Syst. 1999 Nov;15(11-12):652–661. doi: 10.1007/s003810050454. [DOI] [PubMed] [Google Scholar]
- Whitelaw A., Christie S., Pople I. Transforming growth factor-beta1: a possible signal molecule for posthemorrhagic hydrocephalus? Pediatr Res. 1999 Nov;46(5):576–580. doi: 10.1203/00006450-199911000-00014. [DOI] [PubMed] [Google Scholar]
- Wilkinson H. A., Wilson R. B., Patel P. P., Esmaili M. Corticosteroid therapy of experimental hydrocephalus after intraventricular-subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1974 Feb;37(2):224–229. doi: 10.1136/jnnp.37.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]