Abstract
Background: Two devices are available for making transcutaneous estimates of serum bilirubin (SBR): the Minolta AirShields JM102 and the new SpectRx BiliCheck.
Objectives: (a) To measure how well the readings produced by these devices agree with SBR measured in the laboratory; (b) to estimate for each device, the proportion of infants with clinical jaundice who would require blood sampling if the device was used as a screening tool to detect infants with SBR ≥ 250 µmol/l.
Design: Prospective cohort study of jaundiced infants who required SBR at ≤ 20 days of postnatal age. Those who had received phototherapy or exchange transfusion were excluded.
Setting: Tertiary neonatal service in South-East Scotland.
Interventions: Within 30 minutes of SBR sampling, transcutaneous bilirubinometry was performed using one Minolta and two SpectRx devices (designated A and B).
Results: Sixty four neonates were enrolled, 19 of which were preterm (31–35 weeks). The 95% confidence intervals of a device reading corresponding to SBR were ± 66.7, ± 67.9, and ± 66.4 µmol/l respectively. Using the devices to identify all SBR ≥ 250 µmol/l would reduce SBR sampling by 23%, 16%, and 20% respectively.
Conclusions: Given that SBR levels range from 50 to 400 µmol/l in jaundiced infants, the 95% confidence intervals of the devices are wide at ± 67 µmol/l. The SpectRx can be used as a screening tool for hyperbilirubinaemia but there is no advantage in using it over the Minolta.
Full Text
The Full Text of this article is available as a PDF (137.4 KB).
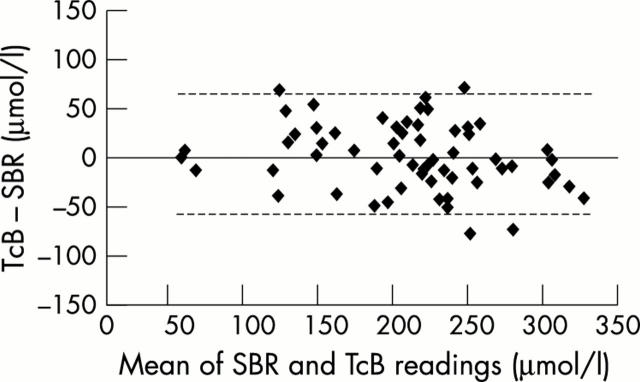
Figure 1 .
Bland-Altman plots for the Minolta device. The dotted line represents 95% confidence intervals of the difference, ± 66.7 µmol/l. SBR, Serum bilirubin; TcB, transcutaneous bilirubinometry.
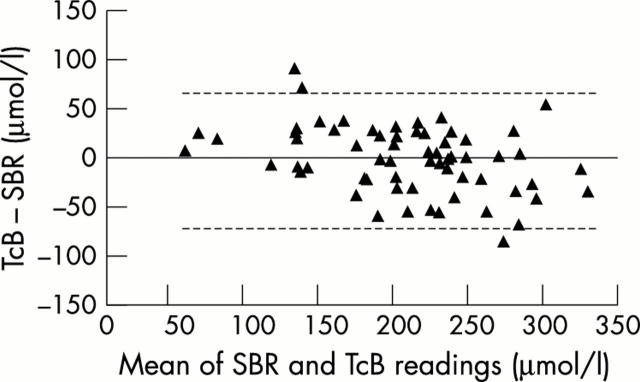
Figure 2 .
Bland-Altman plots for the SpectRx A device. The dotted line represents 95% confidence intervals of the difference, ± 67.9 µmol/l. SBR, Serum bilirubin; TcB, transcutaneous bilirubinometry.
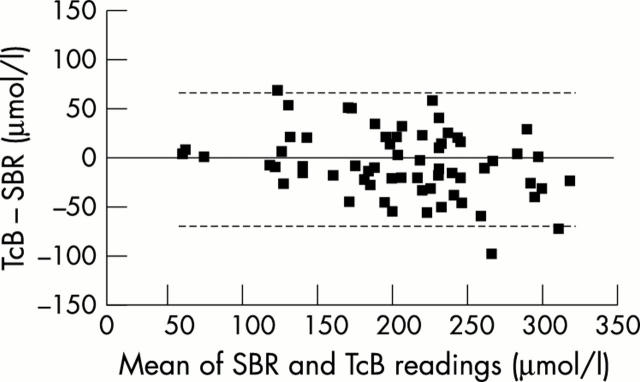
Figure 3 .
Bland-Altman plots for the SpectRx B device. The dotted line represents 95% confidence intervals of the difference, ± 66.4 µmol/l. SBR, Serum bilirubin; TcB, transcutaneous bilirubinometry.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhutani V. K., Gourley G. R., Adler S., Kreamer B., Dalin C., Johnson L. H. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000 Aug;106(2):E17–E17. doi: 10.1542/peds.106.2.e17. [DOI] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. [PubMed] [Google Scholar]
- Hegyi T., Hiatt I. M., Indyk L. Transcutaneous bilirubinometry. I. Correlations in term infants. J Pediatr. 1981 Mar;98(3):454–457. doi: 10.1016/s0022-3476(81)80721-4. [DOI] [PubMed] [Google Scholar]
- Knudsen A., Brodersen R. Skin colour and bilirubin in neonates. Arch Dis Child. 1989 Apr;64(4):605–609. doi: 10.1136/adc.64.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen A., Ebbesen F., Hansen H., Brodersen R. The increase of yellow skin colour beyond that of serum bilirubin: a proposed indicator of risk for bilirubin encephalopathy in the newborn. Acta Paediatr Jpn. 1993 Oct;35(5):418–422. doi: 10.1111/j.1442-200x.1993.tb03084.x. [DOI] [PubMed] [Google Scholar]
- Knudsen A. The cephalocaudal progression of jaundice in newborns in relation to the transfer of bilirubin from plasma to skin. Early Hum Dev. 1990 Apr;22(1):23–28. doi: 10.1016/0378-3782(90)90022-b. [DOI] [PubMed] [Google Scholar]
- Knüpfer M., Pulzer F., Braun L., Heilmann A., Robel-Tillig E., Vogtmann C. Transcutaneous bilirubinometry in preterm infants. Acta Paediatr. 2001 Aug;90(8):899–903. [PubMed] [Google Scholar]
- Madlon-Kay D. J. Recognition of the presence and severity of newborn jaundice by parents, nurses, physicians, and icterometer. Pediatrics. 1997 Sep;100(3):E3–E3. doi: 10.1542/peds.100.3.e3. [DOI] [PubMed] [Google Scholar]
- Moyer V. A., Ahn C., Sneed S. Accuracy of clinical judgment in neonatal jaundice. Arch Pediatr Adolesc Med. 2000 Apr;154(4):391–394. doi: 10.1001/archpedi.154.4.391. [DOI] [PubMed] [Google Scholar]
- O'Leary N., Pembroke A., Duggan P. F. A robust procedure for the automated measurement of total serum bilirubin using potassium ferricyanide. Ann Clin Biochem. 1993 Mar;30(Pt 2):175–179. doi: 10.1177/000456329303000211. [DOI] [PubMed] [Google Scholar]
- Rubaltelli F. F., Gourley G. R., Loskamp N., Modi N., Roth-Kleiner M., Sender A., Vert P. Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics. 2001 Jun;107(6):1264–1271. doi: 10.1542/peds.107.6.1264. [DOI] [PubMed] [Google Scholar]
- Schumacher R. E., Thornbery J. M., Gutcher G. R. Transcutaneous bilirubinometry: a comparison of old and new methods. Pediatrics. 1985 Jul;76(1):10–14. [PubMed] [Google Scholar]
- Tan K. L., Chia H. P., Koh B. C. Transcutaneous bilirubinometry in Chinese, Malay and Indian infants. Acta Paediatr. 1996 Aug;85(8):986–990. doi: 10.1111/j.1651-2227.1996.tb14199.x. [DOI] [PubMed] [Google Scholar]
- Tan K. L., Mylvaganam A. Transcutaneous bilirubinometry in preterm very low birthweight infants. Acta Paediatr Scand. 1988 Nov;77(6):796–801. doi: 10.1111/j.1651-2227.1988.tb10758.x. [DOI] [PubMed] [Google Scholar]
- Tayaba R., Gribetz D., Gribetz I., Holzman I. R. Noninvasive estimation of serum bilirubin. Pediatrics. 1998 Sep;102(3):E28–E28. doi: 10.1542/peds.102.3.e28. [DOI] [PubMed] [Google Scholar]
- Yamanouchi I., Yamauchi Y., Igarashi I. Transcutaneous bilirubinometry: preliminary studies of noninvasive transcutaneous bilirubin meter in the Okayama National Hospital. Pediatrics. 1980 Feb;65(2):195–202. [PubMed] [Google Scholar]
- Yamauchi Y., Yamanouchi I. Transcutaneous bilirubinometry. Evaluation of accuracy and reliability in a large population. Acta Paediatr Scand. 1988 Nov;77(6):791–795. doi: 10.1111/j.1651-2227.1988.tb10757.x. [DOI] [PubMed] [Google Scholar]