Abstract
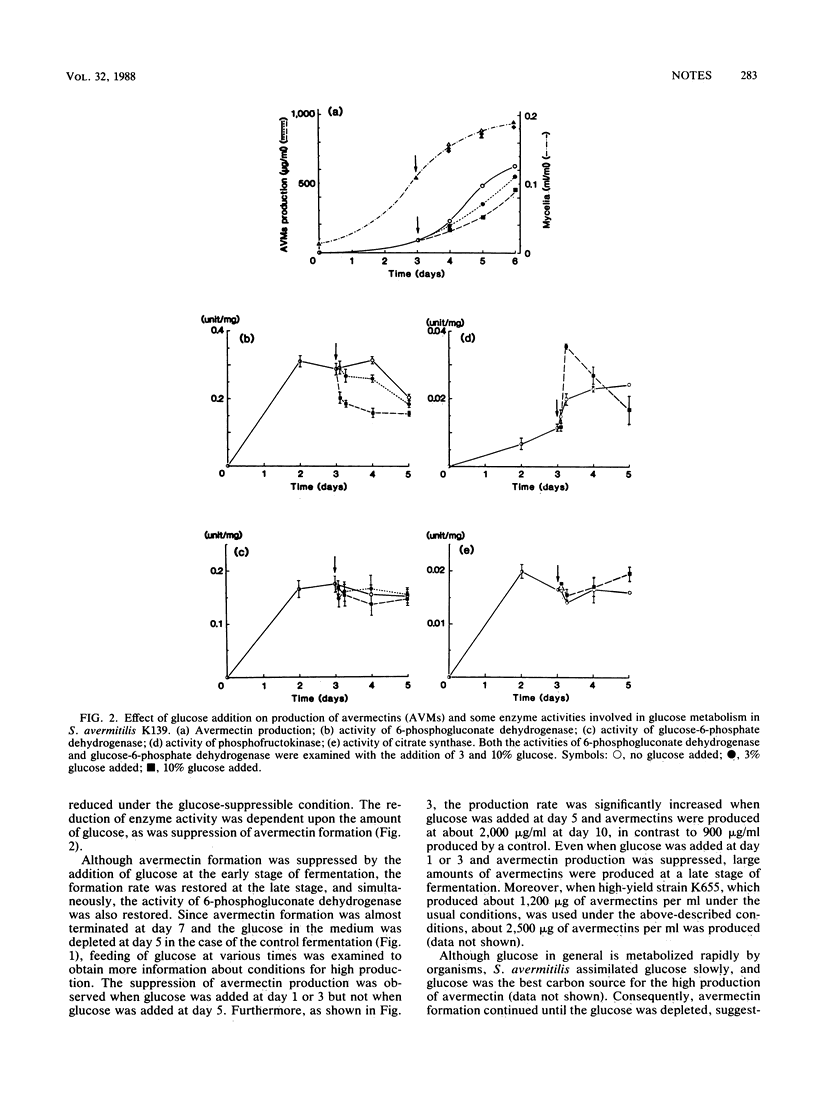
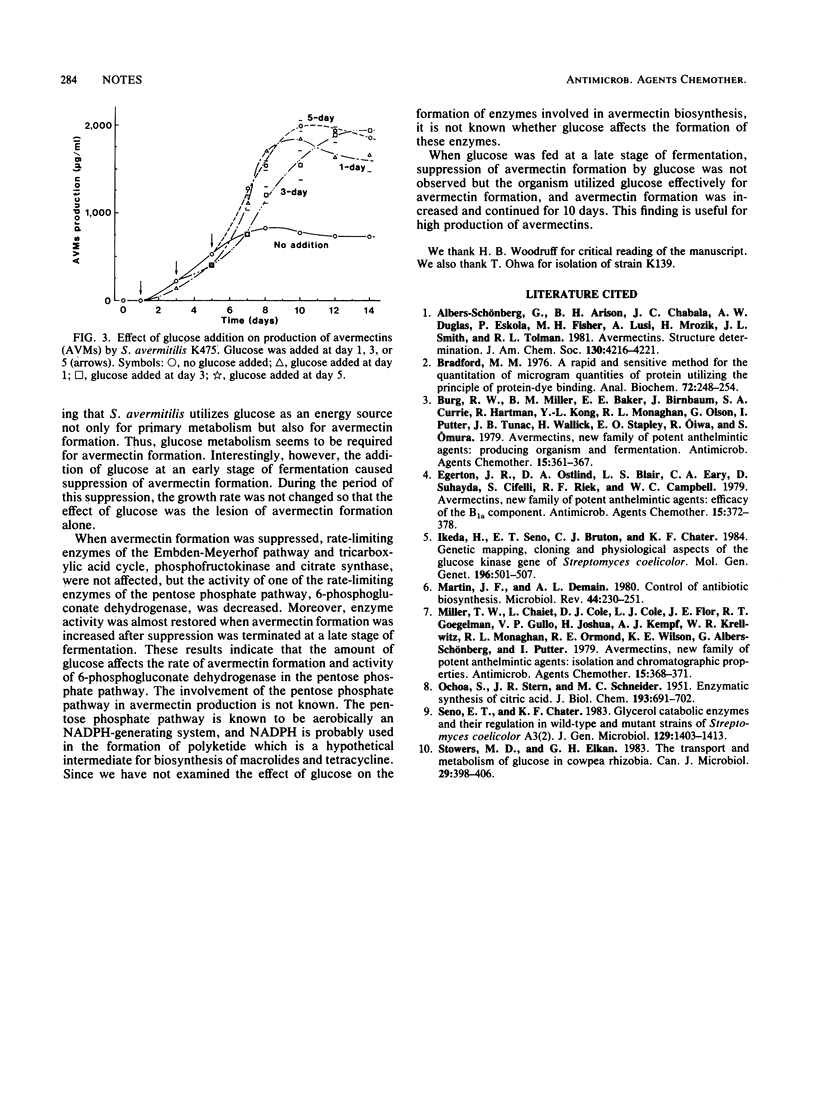
The addition of glucose in the early stage of fermentation suppressed not only avermectin production but also the activity of 6-phosphogluconate dehydrogenase in the pentose phosphate pathway. On the other hand, when glucose was added at the late stage of fermentation, suppression of avermectin formation and 6-phosphogluconate dehydrogenase activity was not observed but avermectin formation was increased and about a twofold-higher content of avermectins than that of the control fermentation was accumulated.
Full text
PDF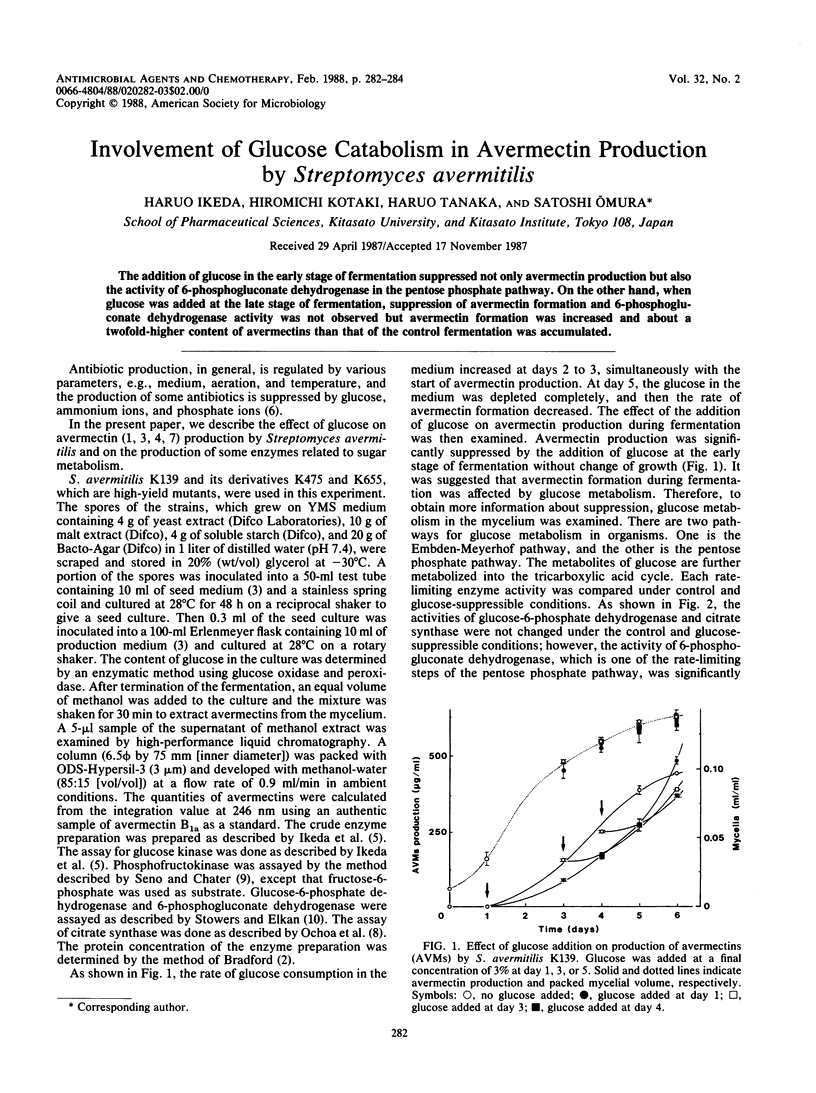
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burg R. W., Miller B. M., Baker E. E., Birnbaum J., Currie S. A., Hartman R., Kong Y. L., Monaghan R. L., Olson G., Putter I. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob Agents Chemother. 1979 Mar;15(3):361–367. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton J. R., Ostlind D. A., Blair L. S., Eary C. H., Suhayda D., Cifelli S., Riek R. F., Campbell W. C. Avermectins, new family of potent anthelmintic agents: efficacy of the B1a component. Antimicrob Agents Chemother. 1979 Mar;15(3):372–378. doi: 10.1128/aac.15.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Seno E. T., Bruton C. J., Chater K. F. Genetic mapping, cloning and physiological aspects of the glucose kinase gene of Streptomyces coelicolor. Mol Gen Genet. 1984;196(3):501–507. doi: 10.1007/BF00436199. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Demain A. L. Control of antibiotic biosynthesis. Microbiol Rev. 1980 Jun;44(2):230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. W., Chaiet L., Cole D. J., Cole L. J., Flor J. E., Goegelman R. T., Gullo V. P., Joshua H., Kempf A. J., Krellwitz W. R. Avermectins, new family of potent anthelmintic agents: isolation and chromatographic properties. Antimicrob Agents Chemother. 1979 Mar;15(3):368–371. doi: 10.1128/aac.15.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCHOA S., STERN J. R., SCHNEIDER M. C. Enzymatic synthesis of citric acid. II. Crystalline condensing enzyme. J Biol Chem. 1951 Dec;193(2):691–702. [PubMed] [Google Scholar]
- Seno E. T., Chater K. F. Glycerol catabolic enzymes and their regulation in wild-type and mutant strains of Streptomyces coelicolor A3(2). J Gen Microbiol. 1983 May;129(5):1403–1413. doi: 10.1099/00221287-129-5-1403. [DOI] [PubMed] [Google Scholar]



