Abstract
Objective: To detail low molecular mass heparin (enoxaparin) use in the first few months of life.
Design: Prospective, consecutive cohort of unselected newborn infants.
Methods: Newborn infants were divided into groups by gestational age, underlying condition, hepatic and renal function, thrombocytopenia, and prothrombin time (PT/INR). Groups were analysed with respect to many aspects of enoxaparin treatment using multivariate methods.
Results: Sixty two newborn infants received enoxaparin representing 5.39 treatment years. Thromboembolic events (TEs) occurred predominantly in the lower and upper venous system in the presence of indwelling catheters (69%). Preterm infants required longer than full term infants to achieve an anti-(factor Xa) level in the target range (six versus two days). Preterm infants required higher doses of enoxaparin than full term infants to maintain anti-(factor Xa) levels in the target range (2.1 v 1.7 mg/kg/12 h). Infants with congenital heart disease (CHD) required less enoxaparin than those without CHD to maintain an anti-(factor Xa) level in the target range (1.7 v 2.1 mg/kg/12 h). Impaired renal and liver function influenced the number of dose changes needed (three versus one a month). Complete or partial resolution of TE was accomplished in 59% of newborn infants. Four infants developed major bleeds (1.2% per patient year). Recurrent TE and clot extension occurred in three infants (0.9% per patient year).
Conclusions: Preterm infants are more difficult to treat with enoxaparin than full term infants. Enoxaparin appears to be an alternative to treatment with standard heparin or no treatment.
Full Text
The Full Text of this article is available as a PDF (122.9 KB).
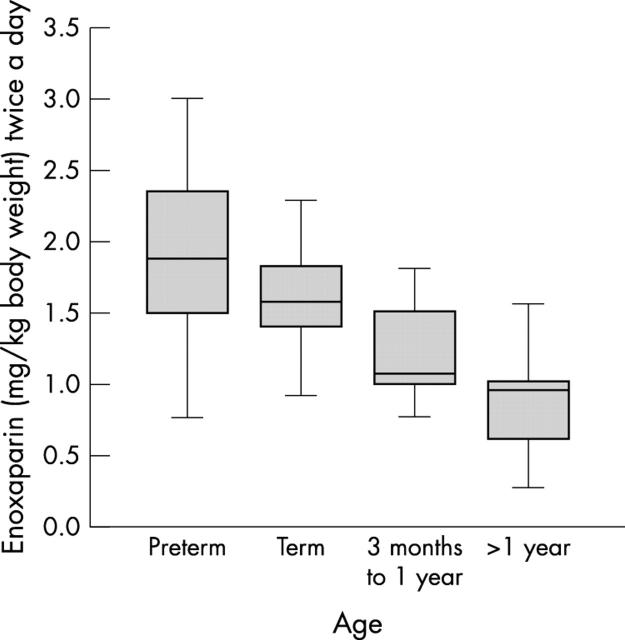
Figure 1.
Effect of gestational age on the dose of enoxaparin required to maintain the anti-(factor Xa) level in the target range of 0.5–1.0 U/ml. Preterm newborn infants required more enoxaparin than full term newborn infants and older children. Data from older children are published elsewhere.14
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew M., Michelson A. D., Bovill E., Leaker M., Massicotte M. P. Guidelines for antithrombotic therapy in pediatric patients. J Pediatr. 1998 Apr;132(4):575–588. doi: 10.1016/s0022-3476(98)70343-9. [DOI] [PubMed] [Google Scholar]
- Andrew M., Paes B., Milner R., Johnston M., Mitchell L., Tollefsen D. M., Castle V., Powers P. Development of the human coagulation system in the healthy premature infant. Blood. 1988 Nov;72(5):1651–1657. [PubMed] [Google Scholar]
- Andrew M., Paes B., Milner R., Johnston M., Mitchell L., Tollefsen D. M., Powers P. Development of the human coagulation system in the full-term infant. Blood. 1987 Jul;70(1):165–172. [PubMed] [Google Scholar]
- Ankola P. A., Atakent Y. S. Effect of adding heparin in very low concentration to the infusate to prolong the patency of umbilical artery catheters. Am J Perinatol. 1993 May;10(3):229–232. doi: 10.1055/s-2007-994726. [DOI] [PubMed] [Google Scholar]
- Dix D., Andrew M., Marzinotto V., Charpentier K., Bridge S., Monagle P., deVeber G., Leaker M., Chan A. K., Massicotte M. P. The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr. 2000 Apr;136(4):439–445. doi: 10.1016/s0022-3476(00)90005-2. [DOI] [PubMed] [Google Scholar]
- Freed M. D., Keane J. F., Rosenthal A. The use of heparinization to prevent arterial thrombosis after percutaneous cardiac catheterization in children. Circulation. 1974 Sep;50(3):565–569. doi: 10.1161/01.cir.50.3.565. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Warkentin T. E., Shaughnessy S. G., Anand S. S., Halperin J. L., Raschke R., Granger C., Ohman E. M., Dalen J. E. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001 Jan;119(1 Suppl):64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- Horgan M. J., Bartoletti A., Polansky S., Peters J. C., Manning T. J., Lamont B. M. Effect of heparin infusates in umbilical arterial catheters on frequency of thrombotic complications. J Pediatr. 1987 Nov;111(5):774–778. doi: 10.1016/s0022-3476(87)80266-4. [DOI] [PubMed] [Google Scholar]
- Levine M. N., Raskob G., Landefeld S., Kearon C. Hemorrhagic complications of anticoagulant treatment. Chest. 1998 Nov;114(5 Suppl):511S–523S. doi: 10.1378/chest.114.5_supplement.511s. [DOI] [PubMed] [Google Scholar]
- Massicotte M. P. Low-molecular-weight heparin therapy in children. J Pediatr Hematol Oncol. 2001 Mar-Apr;23(3):189–194. doi: 10.1097/00043426-200103000-00015. [DOI] [PubMed] [Google Scholar]
- Monagle P., Adams M., Mahoney M., Ali K., Barnard D., Bernstein M., Brisson L., David M., Desai S., Scully M. F. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000 Jun;47(6):763–766. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]
- Monagle P., Michelson A. D., Bovill E., Andrew M. Antithrombotic therapy in children. Chest. 2001 Jan;119(1 Suppl):344S–370S. doi: 10.1378/chest.119.1_suppl.344s. [DOI] [PubMed] [Google Scholar]
- Muir J. M., Andrew M., Hirsh J., Weitz J. I., Young E., Deschamps P., Shaughnessy S. G. Histomorphometric analysis of the effects of standard heparin on trabecular bone in vivo. Blood. 1996 Aug 15;88(4):1314–1320. [PubMed] [Google Scholar]
- Muir J. M., Hirsh J., Weitz J. I., Andrew M., Young E., Shaughnessy S. G. A histomorphometric comparison of the effects of heparin and low-molecular-weight heparin on cancellous bone in rats. Blood. 1997 May 1;89(9):3236–3242. [PubMed] [Google Scholar]
- Punzalan R. C., Hillery C. A., Montgomery R. R., Scott C. A., Gill J. C. Low-molecular-weight heparin in thrombotic disease in children and adolescents. J Pediatr Hematol Oncol. 2000 Mar-Apr;22(2):137–142. doi: 10.1097/00043426-200003000-00011. [DOI] [PubMed] [Google Scholar]
- Saxena A., Gupta R., Kumar R. K., Kothari S. S., Wasir H. S. Predictors of arterial thrombosis after diagnostic cardiac catheterization in infants and children randomized to two heparin dosages. Cathet Cardiovasc Diagn. 1997 Aug;41(4):400–403. doi: 10.1002/(sici)1097-0304(199708)41:4<400::aid-ccd11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Saxon B. R., Black M. D., Edgell D., Noel D., Leaker M. T. Pediatric heparin-induced thrombocytopenia: management with Danaparoid (orgaran). Ann Thorac Surg. 1999 Sep;68(3):1076–1078. doi: 10.1016/s0003-4975(99)00876-0. [DOI] [PubMed] [Google Scholar]
- Severin T., Sutor A. H. Heparin-induced thrombocytopenia in pediatrics. Semin Thromb Hemost. 2001 Jun;27(3):293–299. doi: 10.1055/s-2001-15259. [DOI] [PubMed] [Google Scholar]
- Spadone D., Clark F., James E., Laster J., Hoch J., Silver D. Heparin-induced thrombocytopenia in the newborn. J Vasc Surg. 1992 Feb;15(2):306–312. doi: 10.1067/mva.1992.33807. [DOI] [PubMed] [Google Scholar]
- Streif W., Mitchell L. G., Andrew M. Antithrombotic therapy in children. Curr Opin Pediatr. 1999 Feb;11(1):56–64. doi: 10.1097/00008480-199902000-00011. [DOI] [PubMed] [Google Scholar]
- Sutor A. H., Massicotte P., Leaker M., Andrew M. Heparin therapy in pediatric patients. Semin Thromb Hemost. 1997;23(3):303–319. doi: 10.1055/s-2007-996103. [DOI] [PubMed] [Google Scholar]
- Zöhrer' B., Zenz W., Rettenbacher A., Covi P., Kurnik K., Kroll H., Grubbauer H. M., Muntean W. Danaparoid sodium (Orgaran) in four children with heparin-induced thrombocytopenia type II. Acta Paediatr. 2001 Jul;90(7):765–771. [PubMed] [Google Scholar]