Abstract
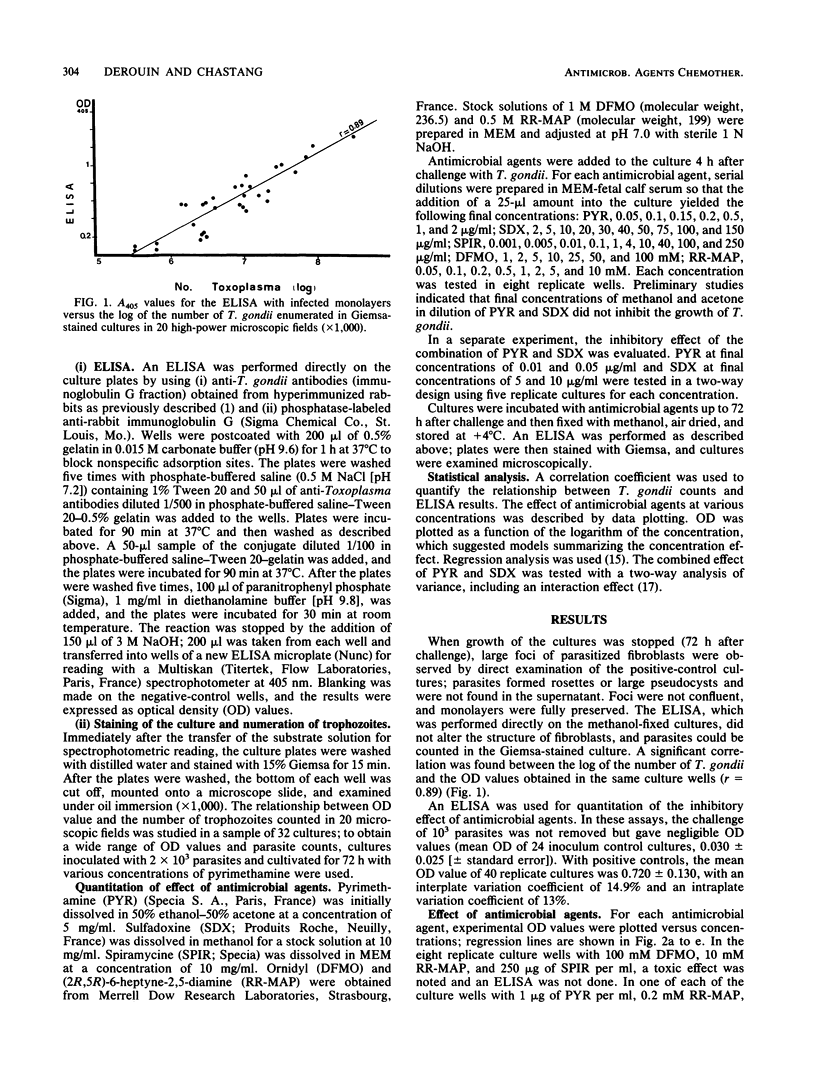
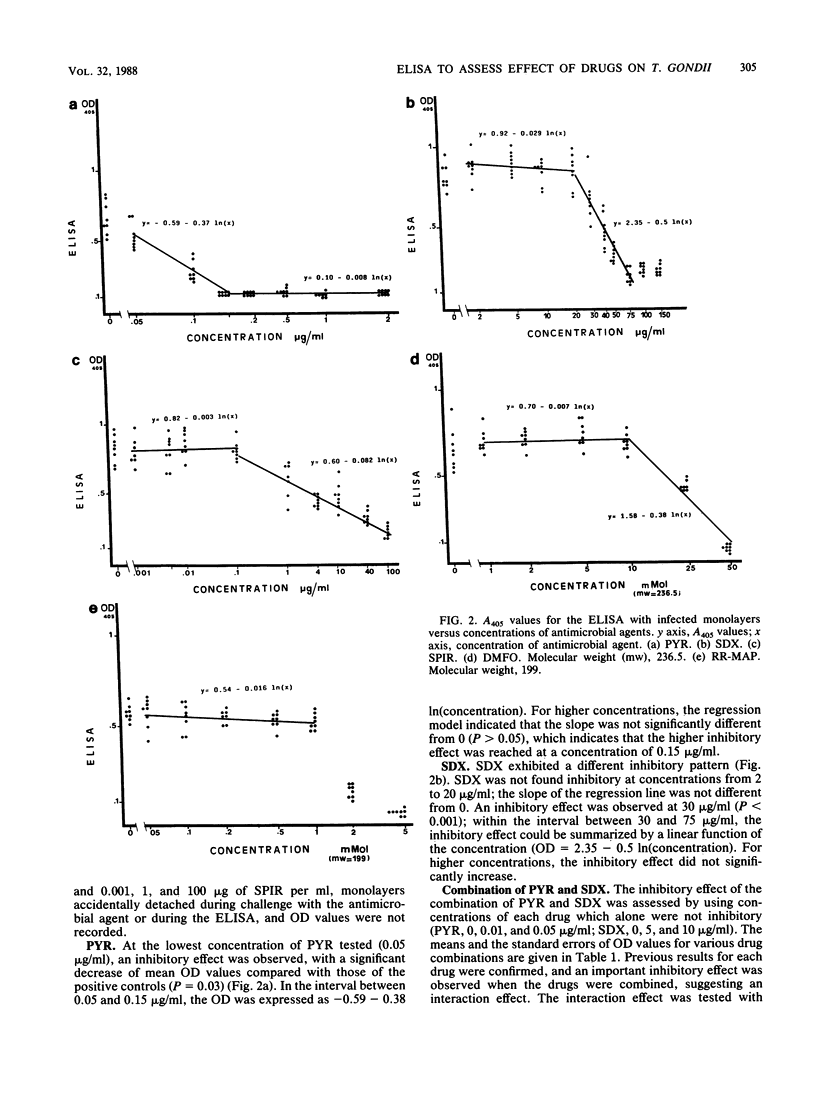
Toxoplasma gondii grown on MRC5 fibroblasts in 96-well tissue culture plates was tested for susceptibility to five antimicrobial agents. T. gondii growth was quantitated by an enzyme-linked immunosorbent assay, which was performed directly on the fixed cultures, using a rabbit anti-T. gondii immunoglobulin G as the first antibody and a phosphatase-labeled anti-rabbit immunoglobulin G as the second antibody. Optical density values were highly correlated with the number of T. gondii organisms in the Giemsa-stained cultures (r = 0.89), and an enzyme-linked immunosorbent assay was used to assess the effect of antimicrobial agents at various concentrations. For each drug, regression models were used to quantify the relationship between optical density values and antimicrobial agent concentrations in the cultures. A significant inhibitory effect was found with pyrimethamine and sulfadoxine for concentrations greater than or equal to 0.05 and 30 micrograms/ml, respectively. With spiramycin, a progressive increase in inhibition of T. gondii was observed for increasing concentrations from 1 to 100 micrograms/ml. Ornidyl (difluoromethylornithine) and (2R,5R)-6-heptyne-2,5-diamine, which are ornithine decarboxylase inhibitors, were found to have a marked inhibitory effect for concentrations greater than or equal to 25 and 2 mM, respectively. This proposed method was sensitive and easy to perform and does not require the use of radiolabeled compounds; since it allows experimental design on replicate cultures and can be partially automated, it thus may prove useful for the systematic screening of the activity of new compounds against T. gondii.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coradello H., Kretschmer S. Vergleichende Untersuchung der Wirksamkeit von Ultrax, Diazil, Baktrim und Spiramycin auf die experimentelle Toxoplasmose der Maus. Wien Klin Wochenschr. 1978 Jan 6;90(1):25–29. [PubMed] [Google Scholar]
- Desmonts G., Couvreur J. Congenital toxoplasmosis. A prospective study of 378 pregnancies. N Engl J Med. 1974 May 16;290(20):1110–1116. doi: 10.1056/NEJM197405162902003. [DOI] [PubMed] [Google Scholar]
- Garin J. P., Paillard B. Toxoplasmose expérimentale de la souris. Activité comparée de: clindamycine, midécamycine, josamycine, spiramycine, pyriméthamine-sulfadoxine, et triméthoprime-sulfaméthoxazole. Ann Pediatr (Paris) 1984 Nov;31(10):841–845. [PubMed] [Google Scholar]
- Garin J. P., Pellerat J., Maillard, Woehrle R., Hezez Bases théoriques de la prévention par la spiramycine de la toxoplasmose congénitale chez la femme enceinte. Presse Med. 1968 Dec 7;76(48):2266–2266. [PubMed] [Google Scholar]
- Gillin F. D., Reiner D. S., McCann P. P. Inhibition of growth of Giardia lamblia by difluoromethylornithine, a specific inhibitor of polyamine biosynthesis. J Protozool. 1984 Feb;31(1):161–163. doi: 10.1111/j.1550-7408.1984.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Grossman P. L., Remington J. S. The effect of trimethoprim and sulfamethoxazole on Toxoplasma gondii in vitro and in vivo. Am J Trop Med Hyg. 1979 May;28(3):445–455. doi: 10.4269/ajtmh.1979.28.445. [DOI] [PubMed] [Google Scholar]
- Hofflin J. M., Guptill D. R., Araujo F. G., Remington J. S. Difluoromethylornithine and formycin B in toxoplasmosis. J Infect Dis. 1985 Nov;152(5):1101–1101. doi: 10.1093/infdis/152.5.1101. [DOI] [PubMed] [Google Scholar]
- Kernbaum S. La spiramycine. Utilisation en thérapeutique humaine. Sem Hop. 1982 Feb 4;58(5):289–297. [PubMed] [Google Scholar]
- Kovacs J. A., Allegra C. J., Chabner B. A., Swan J. C., Drake J., Lunde M., Parrillo J. E., Masur H. Potent effect of trimetrexate, a lipid-soluble antifolate, on Toxoplasma gondii. J Infect Dis. 1987 May;155(5):1027–1032. doi: 10.1093/infdis/155.5.1027. [DOI] [PubMed] [Google Scholar]
- Mack D. G., McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother. 1984 Jul;26(1):26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R., Remington J. S. A method to evaluate the capacity of monocytes and macrophages to inhibit multiplication of an intracellular pathogen. J Immunol Methods. 1979 May 10;27(1):19–29. doi: 10.1016/0022-1759(79)90235-7. [DOI] [PubMed] [Google Scholar]
- Merli A., Canessa A., Melioli G. Enzyme immunoassay for evaluation of Toxoplasma gondii growth in tissue culture. J Clin Microbiol. 1985 Jan;21(1):88–91. doi: 10.1128/jcm.21.1.88-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera P. J., Kramer D. L., Sufrin J. R., Porter C. W. Comparison of the biological effects of four irreversible inhibitors of ornithine decarboxylase in two murine lymphocytic leukemia cell lines. Cancer Res. 1986 Mar;46(3):1148–1154. [PubMed] [Google Scholar]
- Sjoerdsma A., Schechter P. J. Chemotherapeutic implications of polyamine biosynthesis inhibition. Clin Pharmacol Ther. 1984 Mar;35(3):287–300. doi: 10.1038/clpt.1984.33. [DOI] [PubMed] [Google Scholar]



