Abstract
Objective: To assess the cost effectiveness of extracorporeal membrane oxygenation (ECMO) for mature newborn infants with severe respiratory failure over a four year time span.
Design: Cost effectiveness analysis based on a randomised controlled trial in which infants were individually allocated to ECMO (intervention) or conventional management (control) and then followed up to 4 years of age.
Setting: Infants were recruited from 55 approved recruiting hospitals throughout the United Kingdom. Infants allocated to ECMO were transferred to one of five specialist regional centres. Follow up of surviving infants was performed in the community.
Subjects: A total of 185 mature (gestational age at birth ⩾ 35 weeks, birth weight ⩾ 2000 g) newborn infants with severe respiratory failure (oxygenation index ⩾ 40).
Main outcome measures: Incremental cost per additional life year gained; incremental cost per additional disability-free life year gained.
Results: Over four years, the policy of neonatal ECMO was effective at reducing known death or severe disability (relative risk = 0.64; 95% confidence interval 0.47 to 0.86; p = 0.004). After adjustment for censoring and discounting at 6%, the mean additional health service cost of neonatal ECMO was £17 367 (95% confidence interval £12 072 to £22 224) per infant (£UK, 2001 prices). Over four years, the incremental cost of neonatal ECMO was £16 707 (£9828 to £37 924) per life year gained and £24 775 (£13 106 to £69 690) per disability-free life year gained. These results remained robust after variations in the values of key variables performed as part of a sensitivity analysis.
Conclusions: The study provides rigorous evidence of the cost effectiveness of ECMO at four years for mature infants with severe respiratory failure.
Full Text
The Full Text of this article is available as a PDF (196.3 KB).
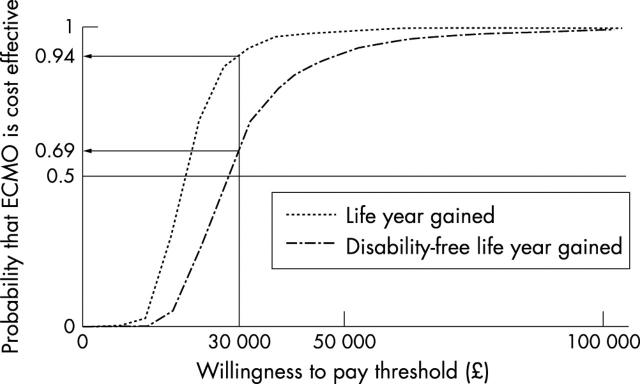
Figure 1 .
Cost effectiveness acceptability curves. Probability that neonatal extracorporeal membrane oxygenation (ECMO) is cost effective after four years plotted as a function of NHS willingness to pay per unit of outcome.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. A., Thompson S. G. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000 Dec 15;19(23):3219–3236. doi: 10.1002/1097-0258(20001215)19:23<3219::aid-sim623>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Bartlett R. H. Extracorporeal life support for cardiopulmonary failure. Curr Probl Surg. 1990 Oct;27(10):621–705. doi: 10.1016/0011-3840(90)90015-w. [DOI] [PubMed] [Google Scholar]
- Bennett C. C., Johnson A., Field D. J., Elbourne D., UK Collaborative ECMO Trial Group UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: follow-up to age 4 years. Lancet. 2001 Apr 7;357(9262):1094–1096. doi: 10.1016/S0140-6736(00)04310-5. [DOI] [PubMed] [Google Scholar]
- Briggs A. H., Gray A. M. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess. 1999;3(2):1–134. [PubMed] [Google Scholar]
- Briggs A., Sculpher M. Sensitivity analysis in economic evaluation: a review of published studies. Health Econ. 1995 Sep-Oct;4(5):355–371. doi: 10.1002/hec.4730040502. [DOI] [PubMed] [Google Scholar]
- Brouwer W., van Hout B., Rutten F. A fair approach to discounting future effects: taking a societal perspective. J Health Serv Res Policy. 2000 Apr;5(2):114–118. doi: 10.1177/135581960000500210. [DOI] [PubMed] [Google Scholar]
- Drummond M. F., Jefferson T. O. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996 Aug 3;313(7052):275–283. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M. Cost-effectiveness guidelines for reimbursement of pharmaceuticals: is economic evaluation ready for its enhanced status? Health Econ. 1992 Jul;1(2):85–92. doi: 10.1002/hec.4730010202. [DOI] [PubMed] [Google Scholar]
- Dworetz A. R., Moya F. R., Sabo B., Gladstone I., Gross I. Survival of infants with persistent pulmonary hypertension without extracorporeal membrane oxygenation. Pediatrics. 1989 Jul;84(1):1–6. [PubMed] [Google Scholar]
- Hallman M., Merritt T. A., Jarvenpaa A. L., Boynton B., Mannino F., Gluck L., Moore T., Edwards D. Exogenous human surfactant for treatment of severe respiratory distress syndrome: a randomized prospective clinical trial. J Pediatr. 1985 Jun;106(6):963–969. doi: 10.1016/s0022-3476(85)80253-5. [DOI] [PubMed] [Google Scholar]
- Laupacis A., Feeny D., Detsky A. S., Tugwell P. X. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992 Feb 15;146(4):473–481. [PMC free article] [PubMed] [Google Scholar]
- Löthgren M., Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ. 2000 Oct;9(7):623–630. doi: 10.1002/1099-1050(200010)9:7<623::aid-hec539>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- O'Neill C., Malek M., Mugford M., Normand C., Tarnow-Mordi W. O., Hey E., Halliday H. L. A cost analysis of neonatal care in the UK: results from a multicentre study. ECSURF Study Group. J Public Health Med. 2000 Mar;22(1):108–115. doi: 10.1093/pubmed/22.1.108. [DOI] [PubMed] [Google Scholar]
- O'Rourke P. P., Crone R. K., Vacanti J. P., Ware J. H., Lillehei C. W., Parad R. B., Epstein M. F. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989 Dec;84(6):957–963. [PubMed] [Google Scholar]
- Parsonage M., Neuburger H. Discounting and health benefits. Health Econ. 1992 Apr;1(1):71–76. doi: 10.1002/hec.4730010110. [DOI] [PubMed] [Google Scholar]
- Peckham M. Research and development for the National Health Service. Lancet. 1991 Aug 10;338(8763):367–371. doi: 10.1016/0140-6736(91)90494-a. [DOI] [PubMed] [Google Scholar]
- Petrou S., Davidson L. L. Economic issues in the follow-up of neonates. Semin Neonatol. 2000 May;5(2):159–169. doi: 10.1053/siny.1999.0005. [DOI] [PubMed] [Google Scholar]
- Petrou Stavros, Murray Lynne, Cooper Peter, Davidson Leslie L. The accuracy of self-reported healthcare resource utilization in health economic studies. Int J Technol Assess Health Care. 2002 Summer;18(3):705–710. doi: 10.1017/s026646230200051x. [DOI] [PubMed] [Google Scholar]
- Raftery J. NICE: faster access to modern treatments? Analysis of guidance on health technologies. BMJ. 2001 Dec 1;323(7324):1300–1303. doi: 10.1136/bmj.323.7324.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. E. Economic evaluation and randomised controlled trial of extracorporeal membrane oxygenation: UK collaborative trial. The Extracorporeal Membrane Oxygenation Economics Working Group. BMJ. 1998 Oct 3;317(7163):911–916. doi: 10.1136/bmj.317.7163.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolar C. J., Snedecor S. M., Bartlett R. H. Extracorporeal membrane oxygenation and neonatal respiratory failure: experience from the extracorporeal life support organization. J Pediatr Surg. 1991 May;26(5):563–571. doi: 10.1016/0022-3468(91)90708-2. [DOI] [PubMed] [Google Scholar]
- Torrance G. W., Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care. 1989;5(4):559–575. doi: 10.1017/s0266462300008461. [DOI] [PubMed] [Google Scholar]
- Trakshel G. M., Sluss P. M., Maines M. D. Comparative effects of tin- and zinc-protoporphyrin on steroidogenesis: tin-protoporphyrin is a potent inhibitor of cytochrome P-450-dependent activities in the rat adrenals. Pediatr Res. 1992 Feb;31(2):196–201. doi: 10.1203/00006450-199202000-00022. [DOI] [PubMed] [Google Scholar]