Abstract
We characterized antigen-specific CD4+ T cells in six patients with treatment-resistant Lyme arthritis, using an HLA-DRB1*0401 major histocompatibility complex (MHC) class II tetramer covalently loaded with OspA164–175, an immunodominant epitope of Borrelia burgdorferi. Direct analysis of OspA-tetramer binding CD4+ cells in patients expressing the HLA-DRB1*0401 allele revealed frequencies of between <0.005 and 0.1% in peripheral blood (n = 6), and between <0.005 and 3.1% in synovial fluid (n = 3). OspA-tetramer+CD4+ cells were directly cloned at 1 cell per well and expanded by mitogen and IL-2 on allogeneic feeder cells. As measured by [3H]thymidine incorporation, 95% of 168 T cell clones from synovial fluid binding the OspA-tetramer were antigen-reactive. Clones generated from peripheral blood revealed a different pattern of responsiveness when compared with clones generated from synovial fluid, as measured by proliferation, IFN-γ, and IL-13 secretion. These clones, selected on the basis of their peptide binding, also responded to whole protein, but with a different cytokine profile. Our studies demonstrate that MHC class II tetramers can be used in humans to directly identify, isolate, and characterize antigen-reactive T cells from an inflammatory compartment.
The recent development of multimeric MHC/peptide complexes with the capacity to bind T cells has provided a tool to study antigen-specific T cell responses directly ex vivo (1–4). Studies using MHC class I tetramers demonstrated that current methods for enumerating antigen-reactive T cells vastly underestimate the size of the antigen-specific, virally induced T cell repertoire (2, 5–7). MHC class I restricted cluster determinant (CD)8+ T cells recognizing microbial antigens have been easily detected in blood, often with frequencies of more than 10% of the CD8+ T cell pool. The generation of MHC class II tetramers recently has allowed the investigation of circulating antigen-specific CD4+ T cells (1, 3, 5, 8). In marked contrast to viral-reactive CD8+ T cells, the frequency of MHC class II tetramers staining antigen-specific CD4+ T cells seems to be significantly lower. Analysis of influenza-specific CD4+ T cells after vaccination in humans indicated that the frequency of antigen-reactive T cells in the circulation was too small to enumerate directly (3). MHC class II DR4 tetramers loaded with the self-antigen collagen II similarly detected a very low frequency of collagen type II autoreactive T cells in patients with rheumatoid arthritis (4).
Previous investigations in human autoimmune diseases have demonstrated the sequestration of antigen-reactive T cells at the site of the inflammation (8). Thus, we chose to analyze antigen-specific T cells with MHC class II tetramers from an inflammatory site, the synovial fluid (SF), of subjects with antibiotic-treatment-resistant Lyme arthritis. This seems to be a particularly good model of a microbial-induced autoimmune disease of the joints that develops in a small percentage of patients in association with infection with Borrelia burgdorferi (Bb). Infection with this tick-borne spirochete induces a complex, multisystem disease in which approximately 60% of untreated patients develop intermittent or chronic oligoarticular arthritis months after disease onset (9–13). It develops while the patient is still infected, but continues after eradication of the spirochete with antibiotic therapy. Approximately 70% of these patients develop high-affinity IgG antibodies to outer-surface protein A (OspA) of Bb near the beginning of prolonged episodes of arthritis (14). Those patients with the HLA-DR4 specificity and OspA reactivity had arthritis for significantly longer after treatment than did those who lacked OspA reactivity. Th1 cells specific for OspA are found primarily in patients with treatment-resistant Lyme arthritis (15, 16). Peptide mapping studies in DRB1*0401 transgenic mice identified a 20-aa region, amino acids 164–183 (OspA164–183), as one of the immunodominant epitopes of OspA (17).
In this study, an HLA-DRB1*0401 MHC class II tetramer covalently loaded with an immunodominant epitope of OspA, OspA164–175, was used to enumerate directly the frequency of OspA-reactive T cells in the SF and peripheral blood mononuclear cells (PBMC) of patients with treatment-resistant Lyme arthritis. Single-cell cloning and subsequent examination of OspA-tetramer+ T cell clones confirmed that the tetramer binding cells ex vivo were highly antigen-reactive. These data demonstrate that MHC class II tetramers can be used to directly enumerate and isolate antigen-reactive T cells in inflamed immune compartments.
Materials and Methods
Patients.
All eight patients met the Centers for Disease Control criteria for the diagnosis of Lyme disease (18). They had swelling of one or both knees accompanied by a positive IgG antibody response to Bb by ELISA and Western blot. Despite antibiotic treatment after the occurrence of arthritis, the joint swelling still persisted, indicating a treatment-resistant course. SF and peripheral blood samples were taken at the same time. At the time of sampling, PCR tests for Bb DNA in joint fluid were negative. Informed consent was obtained from all study subjects.
Preparation of MHC II Tetramers.
Tetramers of peptides from OspA and human synovial matrix glycoprotein (gp)39 bound to human DR4 and of a peptide from mouse cytochrome c (MCC) were prepared as described elsewhere (1, 4). Briefly, soluble MHC class II molecules were prepared by using a two-promoter baculovirus transfer vector (1, 19). The gene encoding the extracellular region of the DR4α chain and the extracellular portion of DR4β chain were cloned behind the p10 promoter and polyhedrin promoter, respectively. Sequence encoding the Bb OspA164–175 or human gp39263–275 peptides and a 14-aa flexible linker were inserted between the leader and the N terminus of the DR4β1 domain. Although the peptide used in T cell hybridoma and clone stimulation was generally OspA164–183, we subsequently found that the important epitope was contained in OspA164–175. Therefore, this shorter peptide was used in the tetramer construction. The C terminus of the DR4β chain carried a peptide tag for biotinylation by the Escherichia coli enzyme BirA (Avidity, Denver, CO). Purified proteins were biotinylated and incorporated into complexes with phycoerythrin/streptavidin (BioSource International, Camarillo, CA; ref. 1).
Production of T Cell Hybridomas (THy).
DR4 transgenic mice were immunized two times with 100 μg of OspA, the first in complete Freund's adjuvant and the second in incomplete Freund's adjuvant. Two weeks after the second immunization, lymph node cells were harvested, pooled, and stimulated with 40 μg/ml OspA in RPMI medium 1640 supplemented with Hepes, glutamine, 2-mercaptoethanol, and penicillin/streptomycin (Biowhittaker, Walkersville, MD) plus 10% FCS. Washed, stimulated lymph node cells were combined with BW-T cell receptor (TCR)−/− cells (American Type Culture Collection) in 35% polyethelene glycol. Fused cells were plated at 1 cell per well and grown in 10% FCS-RPMI medium 1640 supplemented with hypoxanthine–aminopterin–thymidine (Sigma). Lines were weaned from selection media and screened for antigen specificity by incubating THy with irradiated syngeneic spleen cells and OspA164–183 or controls. IL-2 was measured by IL-2-capture ELISA. Growth- and antigen-responsive cell lines were subcloned.
Fluorescent Cell Sorting and T Cell Cloning.
Staining reactions were set up at 300 μl final volume, containing 6–8 × 106 thawed patient SF or PBMC and 20 μg/ml OspA- or control-tetramer (1) in RPMI medium 1640 containing 5% human serum (Gemini Bio-Products, Calabasas, CA), 2 mM glutamine, 10 mM Hepes, 0.1 mM nonessential amino acids (GIBCO/BRL), and 1 mM Na-pyruvate (GIBCO). Reactions were incubated at 37°C for 1.5 h. Anti-CD4-FITC and anti CD64-CyChrome (PharMingen) were added to the reaction mixture for 30 min at 4°C. Samples were washed and resuspended in the same supplemented RPMI medium 1640. Flow cytometric sorting was performed on a FACS Vantage (Becton Dickinson). Only CD64− cells (20) were included in the acquisition. OspA-tetramer+ CD4+CD64− cells were sorted by single cells into 96-well U-bottom plates, containing 200 μl of complete medium and 150,000 irradiated human PBMC (5,000 rads) plus 2 μg/ml phytohemagglutinin, and incubated at 37°C. OspA-tetramer-negative, CD4+CD64− cells also were sorted. At 48 h, 5% T-stim (Collaborative Biomedical Products, Bedford, MA) in complete medium was added to each well. Clones were fed every 3 days with 100 μl of 20 units/ml human recombinant (r) IL-2-containing medium (Teceleukin; National Cancer Institute, Frederick, MD). After 14 days, clones were restimulated with 2 μg/ml phytohemagglutinin and irradiated PBMC. At 48 h, IL-2 was added to wells at a final concentration of 20 units/ml.
Proliferation Assays.
Autologous Epstein–Barr virus (EBV)-transformed B cells were treated with mitomycin-C (Sigma) for use as antigen-presenting cells (APC). Then, 20,000 APC plus antigen at a final concentration of 10 μg/ml plus 2 × 104 cells of each T cell clone were added to wells. At 48 h, half of the medium was removed for cytokine analysis, and plates were pulsed with 0.5 μCi (1 Ci = 37 GBq) [3H]thymidine for 18 h. Plates were counted on a β-plate scintillation counter (EG & G Wallac, Gaithersburg, MD). Screening data are expressed as Δcpm, which is equal to the mean cpm with antigen minus the mean cpm with medium alone. In dose-titration experiments, OspA164–183 was diluted from 10 μg/ml to 1 pg/ml. In some experiments, whole unlipidated rOspA from Bb B31 produced in E. coli was tested. rOspA is approximately 30 kDa. Concentrations of 40 μg/ml and 400 μg/ml of rOspA correspond to a 1.3 μM and 13 μM solutions of protein, respectively. The OspA164–183 peptide at 10 μg/ml is a 4.7 μM solution.
Cytokine ELISA.
Sandwich ELISAs were used to detect IFN-γ production, according to the manufacturer's protocol (PharMingen). Standard curves using human rIFN-γ (GIBCO/BRL) and IL-13 (R & D Systems) were used to quantify cytokine production. Avidin-peroxidase conjugate (Sigma) and 3,3′,5,5′,-tetrametylbenxidine (Kirkegaard & Perry Laboratories) substrate were added sequentially to develop the plates. Substrate development was stopped by using 0.1 M H3PO4, and plates were read at 450 nm. Values were determined by comparing OD450 values to the standard curve.
Tetramer Staining, Dose–Response.
Dilutions of tetramer were added to wells of V-bottom plates containing 5 × 104 cloned cells in 30 μl for final concentrations of 20, 2.0, and 0.2 μg/ml. Reaction mixtures were incubated for 1.5 h at 37°C, and surface marker antibodies were added in the last 30 min. Washed cells were resuspended in 1% FCS/PBS and analyzed by flow cytometry (FACSort; Becton Dickinson).
Intracellular Cytokine Stains.
OspA-reactive T cell clones were stimulated with antigen for a total of 48 h. At 36 h, 2 μM monensin (Sigma) was added; 20 μg/ml OspA-tetramer was added during the last 2 h. Cells were washed, fixed in 4% paraformaldehyde, and washed again in permeabilization buffer (1% FCS/0.1% saponin (Sigma)/0.1% sodium azide in PBS) before staining with anti-cytokine monoclonal antibodies (PharMingen). Anti-IL-4 and anti-IFN-γ and corresponding isotype controls were used. After staining at 4°C for 30 min, cells were washed twice in permeabilization buffer and resuspended in staining buffer for flow cytometric analysis.
Results
Functionality of OspA-Tetramers in THy.
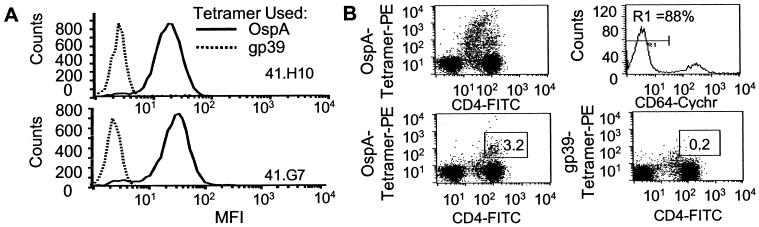
We prepared a panel of THy from lymph node cells of DR4 transgenic (DR4-Tg) mice (21) that had been immunized and boosted with OspA in vivo. Because these mice do not express murine class II molecules, their CD4+ T cells are restricted to the human DR4 molecule. As expected from previous results obtained from bulk T cell cultures (15, 17), the majority of these THy were specific for OspA164–183. Thus, they represented a positive control for the OspA-loaded DR4 tetramer. As can be seen in Fig. 1A, two representative THy bound the OspA-tetramer, but not the control gp39-tetramer, establishing that the OspA-tetramer binds to OspA-specific HLA-DRB1*0401-restricted T cells. Eight other OspA-specific THy bound the OspA-tetramer similarly; and THy that were unresponsive to OspA, but produced IL-2 following CD3 crosslinking, did not bind either tetramer (results not shown).
Figure 1.
OspA-tetramer bind to TCR of THy from DR4-Tg mice and SF T cells from subject 2 with treatment-resistant Lyme arthritis. rOspA immunized DR4-Tg mouse THy (A) and human synovial CD4+ T cells (B) from a subject (2) with treatment-resistant Lyme arthritis were stained with phycoerythrin (PE)-labeled OspA-tetramer (20 μg/ml) and anti-CD4-FITC with only forward- and side scatter used as the lymphocyte gate (Upper Left). Cells gated in addition for lacking binding of anti-CD64-cychrome (R1 in Upper Right) were stained with CD4-FITC and either OspA (Lower Left) or GP39-tetramer (Lower Right).
Visualization and Frequency of OspA-Specific T Cells in Lyme Arthritis Patients.
SF and PBMC from six patients with treatmentresistant Lyme arthritis (HLA-DRB1*0401) were analyzed for OspA164–183-binding T cells by staining with OspA-tetramer. To include both small resting and large activated antigen-specific T cells, the analysis was not restricted with respect to size and granularity of cells. Contaminating monocytes, which nonspecifically bind tetramer, were excluded from analysis by selecting the CD64− [FcγIR− (20)] population (Fig. 1B, gate R1). Staining of PBMC from six patients revealed that only between <0.005 and 0.1% bound the OspA-tetramer, a frequency not significantly higher than that seen with the negative control tetramer. However, in three of the patients in whom concomitant SF samples were available, the frequencies of OspA-tetramer-binding cells in SF were between 0.01 and 3.1% (n = 3) (Table 1). The frequency of T cells binding either of the control tetramers, DRB1*0401-gp-39 or IEk-MCC, were significantly less in SF, between <0.005 and 0.3%, comparable to binding of the OspA-tetramer in patients not expressing DR4. In the SF from a patient homozygous for the HLA-DRB1*0401 allele, 3.1% of the CD4+ cells bound OspA-tetramer, indicating a marked sequestration of OspA-specific cells at the site of joint inflammation (Fig. 1B). Negligible numbers, <0.005–0.1%, of OspA-tetramer binding cells were detected in either SF (n = 1) or PBMC (n = 2) of DRB1*0401-negative patients, confirming the specificity of OspA-tetramer as a staining reagent in DRB1*0401 individuals. No OspA-tetramer staining was seen in the two DRB1*0401 healthy controls.
Table 1.
Frequency analysis of OspA-tetramer-binding CD4+ cells in patients with treatment-resistant Lyme arthritis and in healthy subjects
| Lyme
arthritis
|
Controls
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRB1*0401+
|
DRB1*0401−
|
DRB1*0401+
|
|||||||||||||
| Subject | Synovial fluid
|
Peripheral
blood
|
Subject | Synovial
fluid
|
Peripheral
blood
|
Subject | Peripheral blood
|
||||||||
| % OspA- tetramer | % Control-tetramer | % OspA- tetramer | % Control-tetramer | Ag-Rx clones | % OspA- tetramer | % Control-tetramer | % OspA- tetramer | % Control-tetramer | Ag-Rx clones | % OspA- tetramer | % Control-tetramer | Ag-Rx clones | |||
| 2* | 3.05 | 0.25 | 0.01 | 0.01 | + | 12 | <0.005 | 0.01 | 0.01 | 0.01 | 0 | 11 | 0.02 | <0.005 | 0 |
| 8 | 0.52 | 0.02 | 0.04 | <0.005 | NA | 16 | NA | NA | 0.05 | 0.01 | 0 | 17 | <0.005 | <0.005 | NA |
| 14 | 0.01 | <0.005 | <0.005 | <0.005 | 0 | ||||||||||
| 3 | NA | NA | 0.11 | 0.08 | + | ||||||||||
| 5 | NA | NA | 0.05 | 0.09 | + | ||||||||||
| 10 | NA | NA | <0.005 | 0.01 | 0 | ||||||||||
Ag-Rx clones, + or 0 indicates whether antigen-reactive T cell clones could or could not be generated by single-cell cloning of tetramer-binding T cells, respectively. NA, not analyzed.
Subject is homozygous for HLA-DRB1*0401.
Cloning of OspA-Specific T Cells After Cell Sorting by OspA-Tetramer Staining.
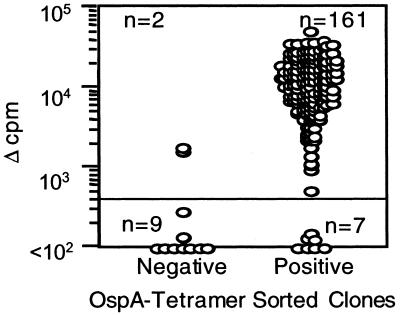
To confirm that T cells identified by the OspA-tetramer staining were indeed antigen-reactive, OspA-tetramer+ T cells were cloned directly from the SF and PBMC of HLA-DRB1*0401+ patients by flow cytometry at 1 cell per well, followed by expansion with phytohemagglutinin and allogeneic feeder cells plus IL-2. Six antigen-specific T cell clones were derived from the PBMC of three patients, whereas 168 clones were generated from the SF of one patient who is homozygous for the DRB1*0401 allele. This finding indicates that it is possible to establish peptide-specific T cell clones, even if the frequency of tetramer-binding CD4+ cells is as low as 0.01%. No clones were generated from PBMC and SF of two HLA-DRB1*0401− patients (Table 1).
The 168 T cell clones derived from the SF of the DRB1*0401 homozygous patient represented a cloning efficiency of 33%. OspA-tetramer− CD4+ clones also were generated from this SF sample to serve as negative controls. All clones were examined for their ability to respond to OspA164–183 (10 μg/ml) in a proliferation assay, measuring [3H]thymidine incorporation. As predicted by their precursor's ability to bind OspA-tetramer, 161/168 of these clones responded to OspA164–183, presented by autologous EBV-transformed B cells (Fig. 2). On the other hand, only 2/11 of the T cell clones derived from the OspA-tetramer−CD4+ fraction responded in the proliferation assay to the OspA peptide (Fig. 2). In 33/161 OspA-reactive clones, the IFN-γ secretion in response to OspA also was analyzed after 48 h of culture. All 33 clones secreted >700 pg/ml IFN-γ (data not shown).
Figure 2.
Human T cell clones generated from OspA-tetramer-sorted SF proliferate in response to OspA164–183. Each of the 168 T cell clones generated from SF from patient 2 was tested in a split-well assay with OspA164–183 and autologous EBV B cells as APC. After 72 h, proliferation was measured as [3H]thymidine uptake.
Dose Titration of OspA-Reactive Clones.
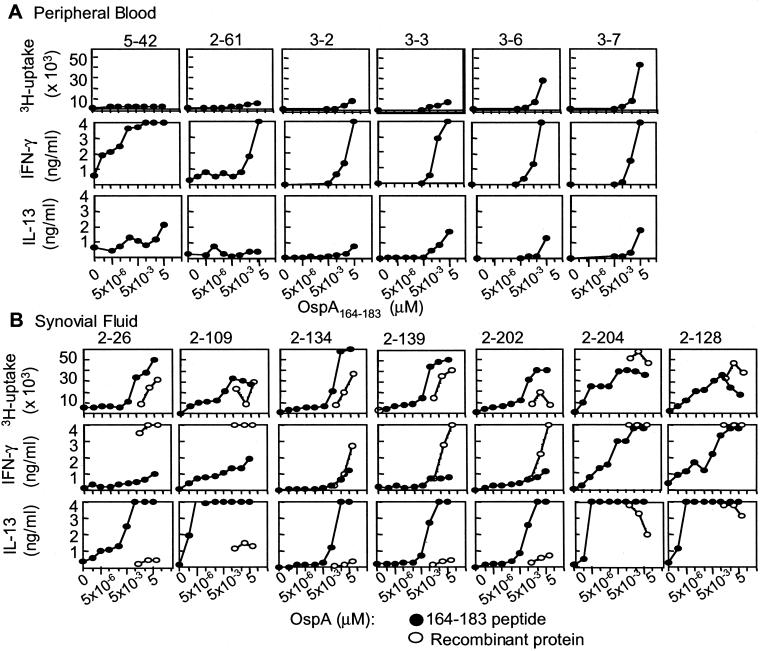
To characterize the dose–response to the cognate peptide antigen, dose titrations were performed on a panel of established clones, and their proliferation and cytokine secretion were evaluated. OspA-reactive T cell lines derived from PBMC proliferated poorly in response to peptide, but secreted high amounts of IFN-γ (Fig. 3A). All clones also secreted IL-13. Interestingly, clones established from SF revealed a different pattern of responsiveness (Fig. 3B), with a higher proliferative response to low concentrations of OspA peptide. Additionally, the SF-derived T cells secreted high amounts of IL-13, with relatively low amounts of IFN-γ (Fig. 3B).
Figure 3.
Dose-dependent proliferative and cytokine responses to OspA. A selection of clones was tested in a split-well assay for [3H]thymidine uptake and cytokine secretion. Clones from PBMC (patients 2, 3, and 5) (A) and clones from SF (patient 2) (B) were tested with concentrations of OspA164–183 ranging from 1 pg/ml to 10 μg/ml (filled circles) (10 μg/ml equivalent to 4.7 μM) and with rOspA protein at 4, 40, and 400 μg/ml (open circles) (40 μg/ml equivalent to 1.3 μM). Cell culture supernatants were analyzed at 48 h of culture for IFN-γ and IL-13; [3H]thymidine uptake was measured after 72 h.
It was important to demonstrate that T cells cloned by selection with the OspA-tetramer represent cells in vivo that can recognize whole OspA processed by APC. For this purpose, OspA peptide-reactive T cell clones were tested with purified rOspA and autologous EBV-transformed B cells. The majority of the T cell clones recognized the whole rOspA on an equimolar basis as compared with peptide, when the proliferative responses were analyzed (Fig. 3B). However, with respect to cytokine secretion, rOspA induced high levels of IFN-γ and low levels of IL-13 after 48 h of culture. This finding is in contrast to the cytokine pattern observed in the clones after stimulation with peptide, where high amounts of IL-13 and low amounts of IFN-γ were secreted.
OspA-Responsive Clones Bind OspA-Tetramer.
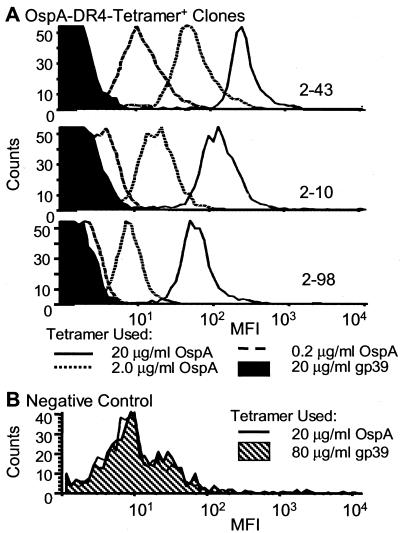
Individual T cell clones were characterized by flow cytometry for binding to the MHC class II tetramers (Fig. 4). When the OspA-reactive clones were stained with 0.2, 2, and 20 μg/ml OspA-tetramer for 2 h at 37°C, a concentration-dependent increase in the mean fluorescence intensity (MFI) was observed (Fig. 4A). At concentrations of 2 μg/ml, all cells within a clone bound the tetramer with an MFI in the range of 5 × 101 to 3 × 102. With lower OspA-tetramer concentrations, only a few clones showed a staining pattern distinctly different from that of the control tetramer. This concentration-dependent shift in MFI did not occur with increasing concentrations of control gp39-tetramer (data not shown) or with OspA-tetramer sort negative clones stained with the OspA-tetramer (Fig. 4B).
Figure 4.
Dose titration of OspA-tetramer on OspA-specific T cell clones. (A) Representative clones were stained with 0.2, 2, and 20 μg/ml of OspA-tetramer for 2 h at 37°C before flow cytometry analysis. (B) As a negative control, a CD4+ T cell clone not responding to OspA in vitro was used.
Tetramer Staining Can Be Combined with Intracellular Cytokine Staining.
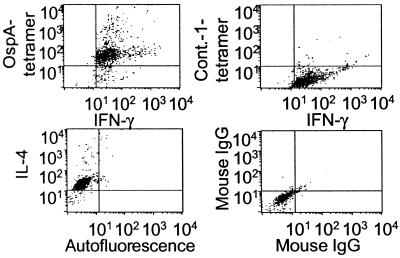
To analyze cytokine production of antigen-specific T cells at the single-cell level, OspA-tetramer and intracellular cytokine stains were combined. OspA-reactive clones were activated for 48 h, with the addition of monensin for the last 12 h. Tetramer staining was performed at 37°C for the last 2 h of culture. All cells within the clone bound the tetramer and exhibited intracytoplasmic expression of IFN-γ (Fig. 5). The few cells that were clearly brighter in their stain for OspA-tetramer or IFN-γ could not be distinguished with regard to their size or granularity (data not shown). All cells within a clone also expressed intracytoplasmic IL-4 (Fig. 5).
Figure 5.
Tetramer staining combined with intracellular cytokine staining on a representative OspA-reactive T cell clone. T cell clone was activated in vitro for 48 h; at 36 h, monensin was added; then during the last 2 h, 20 μg/ml OspA or control (LFA-1) tetramer was added. Cells were fixed, stained with directly labeled antibodies specific for IFN-γ, IL-4, and isotype controls for 30 min at 4°C, and washed twice before fluorescence-activated cell sorter (FACS) analysis. This clone did not respond to LFA-1 in in vitro proliferation assays.
Discussion
Here we demonstrate that MHC class II tetramers can be used for the direct enumeration of antigen-reactive CD4+ T cells in a microbial-induced human disease. A frequency of up to 1 in 30 of OspA-tetramer+CD4+-CD64-depleted T cells were identified in the inflamed joint of patients with antibiotic-treatment-resistant Lyme arthritis, whereas a frequency of less than 1 in 1,000 OspA-tetramer+CD4+ T cells were detected in PBMC. T cell clones derived from OspA-tetramer+ T cells respond functionally to OspA in vitro by proliferation and secretion of IFN-γ. Thus, MHC class II tetramers can be used ex vivo to directly identify antigen-reactive T cells.
Of the SF samples analyzed from three patients, one showed a significantly higher frequency of OspA-tetramer+ T cells as compared with the other two subjects. Interestingly, this patient is homozygous for the HLA-DRB1*0401 allele. Analysis of larger patient populations will be required to determine whether homozygous expression of the DRB1*0401 allele is associated with this marked increase in OspA-reactive T cells.
The OspA-tetramers clearly identified the expected antigen-specific CD4+ T cells in individuals carrying the HLA-DRB1*0401 allele. The parallel analysis of SF and PBMC suggests the importance of examining T cells from inflamed compartments, as only a few OspA-reactive cells were found in the circulation. This result is in striking contrast to the high frequency of circulating antigen-specific CD8+ T cells observed after viral infections (5, 7). Previous reports examining CD4+ T cell responses to the influenza vaccine (3) and our present study indicate that the frequency of circulating CD4+ T cells responding to exogenous antigen may be appreciably lower than that of CD8+ T cells. There are a number of possible explanations for the differences in frequency between CD4+ and CD8+ T cells. First, there might be distinct compartments where CD4+ and CD8+ cells migrate, ultimately associated with lower number of circulating CD4+ cells. Alternatively, the high frequency of CD8+ T cells may represent terminally differentiated cytotoxic effector cells that will soon undergo apoptosis, as opposed to long-term memory CD4+ or CD8+ T cells. Also possible is a more trivial explanation, such as different propensities for down-regulation of the TCR upon activation, where the T cell would become invisible to tetramers (22).
The high frequency of antigen-reactive T cell clones (161/168) generated from OspA-tetramer+CD4+ SF cells confirms that binding of tetramer to CD4+ T cells is antigen-specific. A higher frequency of OspA-tetramer binding T cells was observed in the SF as compared with blood. Additionally, peptide dose titration measurements indicate that the T cell clones generated from SF are more sensitive to antigen stimulation with low antigen concentrations than are T cell clones analyzed from peripheral blood. These data suggest that higher-affinity T cells were captured with the OspA-tetramer from SF than PBMC, consistent with the hypothesis that high-affinity OspA-reactive T cells are being sequestered in the joint tissue in response to an antigen-specific signal. Moreover, because the frequency of tetramer-binding cells was low in PBMC, the sorting gates were wider than those of the SF, allowing the inclusion of tetramer-binding cells with low fluorescent intensity. Nevertheless, we were able to clone antigen-reactive T cells from blood, even when OspA-tetramers revealed frequencies of <0.01% of the CD4+ population. These results indicate that events captured by flow cytometry with OspA-tetramer binding of PBMC T cells represent cognate interactions with the TCR.
Interestingly, the OspA-reactive clones established from SF show a different pattern of responsiveness than do the clones established from PBMC, as measured by levels of proliferation, IFN-γ, and IL-13 secretion. This finding may be attributable to antigen priming of the cells in different compartments, possibly with different APC. Comparing the response pattern of the SF T cells to peptide and rOspA also reveals differences, possibly pointing to differential presentation of naturally processed versus synthetic peptide, which would provide a possible explanation for the different patterns seen for the peptide-reactive clones established from SF and PBMC.
As expected, the OspA-tetramer bound to antigen-reactive T cell clones in a dose-dependent manner, as measured by MFI. Crawford et al. (1) reported similar results with the IEk-MCC tetramers on mouse THy specific for that same MCC peptide. It is possible that the use of even higher concentrations of OspA-tetramer would cause a further increase in the MFI, which would indicate that the binding, as measured, is not at saturation. Similarly to the MCC THy system, increased MFI associated with increased concentrations of OspA-tetramer varied between individual T cell clones. The interclonal differences may be an indication of the number of TCR molecules expressed on each clone or of the affinity of the various TCR for the peptide (1). This is an important question, which will require further analysis of clonal TCR usage. However, even if the tetramer staining is not at saturation, the uniform staining of all cells within a clone clearly demonstrates that even limiting amounts of tetramer can distinguish antigen-specific from non-antigen-specific cells. In contrast to the effects of varying antigen concentration, the DR4 tetramer loaded with the weaker lymphocyte function-associated antigen (LFA)-1332–345 peptide agonist binds only a small fraction of the OspA/LFA-1 dual-reactive T cells clones (unpublished data). Thus, as predicted from recent experiments examining the nature of the “immunological synapse” (23), there are both quantitative and qualitative differences in the engagement of MHC/peptide-tetramers on the surface of T cells.
Two T cell clones of 11 derived from the OspA-tetramer-negative CD4+ population proliferated in response to OspA. This finding may simply reflect a mechanical error in cell sorting. They may also represent OspA-reactive T cells that were recently activated in vivo, resulting in down-regulation of their TCR. Lastly, as discussed previously, low-affinity TCR recognizing OspA may not efficiently bind the OspA-tetramer. Such T cells, when cloned, could respond to antigen presented in the context of strong costimulatory signals, as provided by EBV-transformed B cells. In support of this last idea, when one of these OspA-reactive clones was subsequently tested for binding the OspA-tetramer, it was still negative. Thus, the frequency of OspA-reactive T cells in SF may even be higher than that detected by the OspA-tetramer used in this study.
These experiments demonstrate that MHC class II tetramers can be used to directly enumerate antigen-reactive T cells ex vivo. However, unlike microbial-reactive CD8+ T cell populations that are easily identified in the circulation, direct enumeration of antigen-reactive CD4 T cells may require examination of inflamed tissue sites. Combination of labeled MHC/antigen tetramers with intracellular cytokine analysis will allow the direct functional characterization of antigen-reactive T cells in human disease.
Acknowledgments
We thank Maris Handley at the Dana–Farber flow cytometry facility for excellent help with single-cell sorting, Theresa Willett for providing us with rOspA, Dr. Lee-Ann Baxter-Lowe for the HLA typing of the study patients, and members of the Huber and Hafler labs for reviewing the manuscript. This work was supported by grants from the National Institutes of Health [AR-45386 (to B.T.H.), AR-20358 (to A.C.S.), AR-08541 (to A.L.M.), AI-17134 (to J.K and P.M.), and NS2424710, AI39671, and NS38037 (to D.A.H.)] and Training Grant AR-07570 (to A.L.M.). Other support was from the Arthritis Foundation (B.T.H. and A.C.S.), the National Multiple Sclerosis Society Grants RG2172B6 and RG2949A (to D.A.H.), the Mathers Foundation (A.C.S. and B.T.H.), the Eshe Fund (A.C.S. and B.T.H.), The Swedish Medical Research Council, The Swedish Institute, and The Swedish Medical Society (C.T.).
Abbreviations
- Bb, Borrelia burgdorferi
EBV, Epstein–Barr virus
- MCC
mouse cytochrome c
- OspA
outer surface protein A
- MFI
mean fluorescence intensity
- PBMC
peripheral blood mononuclear cell
- r
recombinant
- SF
synovial fluid
- THy
T cell hybridomas
- TCR
T cell receptor
- APC
antigen-presenting cells
- LFA
lymphocyte function-associated antigen
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190335897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190335897
References
- 1.Crawford F, Kozono H, White J, Marrack P, Kappler J. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 2.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 3.Novak E J, Liu A W, Nepom G T, Kwok W W. J Clin Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotzin B L, Falta M T, Crawford F, Rosloniec E F, Bill J, Marrack P, Kappler J. Proc Natl Acad Sci USA. 2000;97:291–296. doi: 10.1073/pnas.97.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 6.Bieganowska K, Hollsberg P, Buckle G J, Lim D G, Greten T F, Schneck J, Altman J D, Jacobson S, Ledis S L, Hanchard B, et al. J Immunol. 1999;162:1765–1771. [PubMed] [Google Scholar]
- 7.Tan L C, Gudgeon N, Annels N E, Hansasuta P, O'Callaghan C A, Rowland-Jones S, McMichael A J, Rickinson A B, Callan M F. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 8.Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner H L, Hafler D A. J Exp Med. 1994;179:973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steere A C, Grodzicki R L, Kornblatt A N, Craft J E, Barbour A G, Burgdorfer W, Schmid G P, Johnson E, Malawista S E. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 10.Steere A C, Schoen R T, Taylor E. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 11.Steere A C. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 12.Steere A C, Levin R E, Molloy P J, Kalish R A, Abraham J H, 3rd, Liu N Y, Schmid C H. Arthritis Rheum. 1994;37:878–888. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- 13.Steere A C. In: Lyme Disease. Rahn D W, Evans J, editors. Philadelphia: Am. College Physicians; 1998. pp. 107–122. [Google Scholar]
- 14.Kalish R A, Leong J M, Steere A C. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross D M, Steere A C, Huber B T. J Immunol. 1998;160:1022–1028. [PubMed] [Google Scholar]
- 16.Chen J, Field J A, Glickstein L, Molloy P J, Huber B T, Steere A C. Arthritis Rheum. 1999;42:1813–1822. doi: 10.1002/1529-0131(199909)42:9<1813::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Gross D M, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy Z A, Field J A, Steere A C, Huber B T. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Morbid Mortal Wkly Rep. 1997;46:20. [Google Scholar]
- 19.Kozono H, White J, Clements J, Marrack P, Kappler J. Nature (London) 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- 20.Macey M G, McCarthy D A, Vogiatzi D, Brown K A, Newland A C. Cytometry. 1998;31:199–207. doi: 10.1002/(sici)1097-0320(19980301)31:3<199::aid-cyto7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21.Ito K, Bian H J, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin D R, Arceo R, Campbell R, Falcioni F, et al. J Exp Med. 1996;183:2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh Y, Hemmer B, Martin R, Germain R N. J Immunol. 1999;162:2073–2080. [PubMed] [Google Scholar]
- 23.Grakoui A, Bromley S K, Sumen C, Davis M M, Shaw A S, Allen P M, Dustin M L. Science. 1999;285:221–227. [PubMed] [Google Scholar]