Abstract
Background: The incidence of neonatal abstinence syndrome (NAS) has increased 10-fold over the last decade in Glasgow. In the Princess Royal Maternity Hospital, it now accounts for 17% of special care baby unit (SCBU) admissions.
Objective: To compare opiate replacement therapy (morphine sulphate) with the present standard treatment (phenobarbitone) for management of NAS. The primary study end point was duration of pharmaceutical treatment. Secondary end points were the requirement for additional drugs and the requirement for SCBU admission.
Design: Double blind, randomised controlled clinical trial.
Methods: Differential diagnoses were excluded, and two consecutive Lipsitz scores > 4 defined NAS requiring treatment. Infants were randomised to receive morphine sulphate or phenobarbitone. Treatments were identical in appearance, odour, and volume. Increments, decrements, and discontinuation of treatments were protocol driven.
Results: Seventy five infants participated. All mothers received opiate replacement therapy (methadone) during pregnancy and most used other drugs (n = 62, 83%). No significant difference in maternal drug use patterns was observed between treatment groups. Median treatment duration was four days shorter with opiate replacement (8 v 12 days, Mann-Whitney U test, p = 0.02). Phenobarbitone treated infants tended to require second line treatment (47% v 35%, χ2 test, p = 0.11) and SCBU admission (62% v 30%, χ2 test, p = 0.04) more often.
Conclusions: Opiate replacement therapy appears to be superior for management of symptomatic NAS when maternal opiate use is prevalent. The shorter treatment duration and lower requirement for higher intensity nursing may have significant cost implications. Tailoring NAS treatment to local maternal drug use may result in similar benefits.
Full Text
The Full Text of this article is available as a PDF (187.7 KB).
Figure 1.
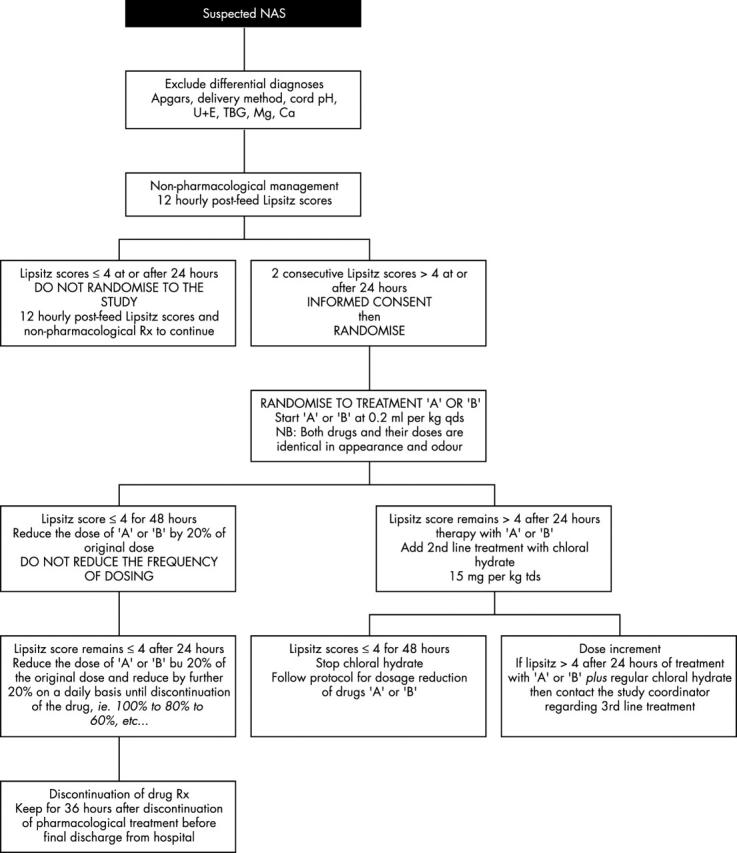
Flow chart summarising the study protocol for start, discontinuation, and dosage adjustment of treatments. NAS, Neonatal abstinence syndrome; TBG, true blood glucose; Rx, prescription; U+E, urea and electrolytes.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alroomi L. G., Davidson J., Evans T. J., Galea P., Howat R. Maternal narcotic abuse and the newborn. Arch Dis Child. 1988 Jan;63(1):81–83. doi: 10.1136/adc.63.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberczak T. M., Kandall S. R., Friedmann P. Relationship between maternal methadone dosage, maternal-neonatal methadone levels, and neonatal withdrawal. Obstet Gynecol. 1993 Jun;81(6):936–940. [PubMed] [Google Scholar]
- Farkas A. G., Colbert D. L., Erskine K. J. Anonymous testing for drug abuse in an antenatal population. Br J Obstet Gynaecol. 1995 Jul;102(7):563–565. doi: 10.1111/j.1471-0528.1995.tb11363.x. [DOI] [PubMed] [Google Scholar]
- Finnegan L. P., Connaughton J. F., Jr, Kron R. E., Emich J. P. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2(1-2):141–158. [PubMed] [Google Scholar]
- Finnegan L. P., Mitros T. F., Hopkins L. E. Management of neonatal narcotic abstinence utilizing a phenobarbital loading dose method. NIDA Res Monogr. 1979;27:247–253. [PubMed] [Google Scholar]
- Green M., Suffet F. The Neonatal Narcotic Withdrawal Index: a device for the improvement of care in the abstinence syndrome. Am J Drug Alcohol Abuse. 1981;8(2):203–213. doi: 10.3109/00952998108999125. [DOI] [PubMed] [Google Scholar]
- Hoder E. L., Leckman J. F., Poulsen J., Caruso K. A., Ehrenkranz R. A., Kleber H. D., Cohen D. J. Clonidine treatment of neonatal narcotic abstinence syndrome. Psychiatry Res. 1984 Nov;13(3):243–251. doi: 10.1016/0165-1781(84)90039-8. [DOI] [PubMed] [Google Scholar]
- Kaltenbach K., Finnegan L. P. Neonatal abstinence syndrome, pharmacotherapy and developmental outcome. Neurobehav Toxicol Teratol. 1986 Jul-Aug;8(4):353–355. [PubMed] [Google Scholar]
- Kandall S. R. Treatment strategies for drug-exposed neonates. Clin Perinatol. 1999 Mar;26(1):231–243. [PubMed] [Google Scholar]
- Khoo B. H. Neonatal narcotic drug withdrawal syndrome. Med J Malaysia. 1978 Jun;32(4):297–301. [PubMed] [Google Scholar]
- Lipsitz P. J. A proposed narcotic withdrawal score for use with newborn infants. A pragmatic evaluation of its efficacy. Clin Pediatr (Phila) 1975 Jun;14(6):592–594. doi: 10.1177/000992287501400613. [DOI] [PubMed] [Google Scholar]
- Madden J. D., Chappel J. N., Zuspan F., Gumpel J., Mejia A., Davis R. Observation and treatment of neonatal narcotic withdrawal. Am J Obstet Gynecol. 1977 Jan 15;127(2):199–201. doi: 10.1016/s0002-9378(16)33250-1. [DOI] [PubMed] [Google Scholar]
- Morrison C. L., Siney C. A survey of the management of neonatal opiate withdrawal in England and Wales. Eur J Pediatr. 1996 Apr;155(4):323–326. doi: 10.1007/BF02002721. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Michailevskaya V., Lukashov I., Bar-Hamburger R., Harel S. The developmental outcome of children born to heroin-dependent mothers, raised at home or adopted. Child Abuse Negl. 1996 May;20(5):385–396. doi: 10.1016/0145-2134(96)00014-2. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Segal J., Bar-Hamburger R., Greenbaum C. Developmental outcome of school-age children born to mothers with heroin dependency: importance of environmental factors. Dev Med Child Neurol. 2001 Oct;43(10):668–675. doi: 10.1017/s0012162201001219. [DOI] [PubMed] [Google Scholar]
- Osborn D. A., Cole M. J., Jeffery H. E. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2002;(3):CD002059–CD002059. doi: 10.1002/14651858.CD002059. [DOI] [PubMed] [Google Scholar]
- Osborn D. A., Jeffery H. E., Cole M. J. Sedatives for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2002;(3):CD002053–CD002053. doi: 10.1002/14651858.CD002053. [DOI] [PubMed] [Google Scholar]
- Ostrea E. M., Jr, Brady M., Gause S., Raymundo A. L., Stevens M. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992 Jan;89(1):107–113. [PubMed] [Google Scholar]
- Ostrea E. M., Jr, Knapp D. K., Tannenbaum L., Ostrea A. R., Romero A., Salari V., Ager J. Estimates of illicit drug use during pregnancy by maternal interview, hair analysis, and meconium analysis. J Pediatr. 2001 Mar;138(3):344–348. doi: 10.1067/mpd.2001.111429. [DOI] [PubMed] [Google Scholar]
- Pacifico P., Nardelli E., Pantarotto M. F. Neonatal heroin withdrawal syndrome; evaluation of different pharmacological treatments. Pharmacol Res. 1989 Nov-Dec;21 (Suppl 1):63–64. doi: 10.1016/s1043-6618(89)80053-2. [DOI] [PubMed] [Google Scholar]
- Rivers R. P. Neonatal opiate withdrawal. Arch Dis Child. 1986 Dec;61(12):1236–1239. doi: 10.1136/adc.61.12.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. J., McIvor L. Neonatal abstinence syndrome after maternal methadone treatment. Arch Dis Child Fetal Neonatal Ed. 1994 Nov;71(3):F203–F205. doi: 10.1136/fn.71.3.f203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood R. A., Keating J., Kavvadia V., Greenough A., Peters T. J. Substance misuse in early pregnancy and relationship to fetal outcome. Eur J Pediatr. 1999 Jun;158(6):488–492. doi: 10.1007/s004310051126. [DOI] [PubMed] [Google Scholar]
- Theis J. G., Selby P., Ikizler Y., Koren G. Current management of the neonatal abstinence syndrome: a critical analysis of the evidence. Biol Neonate. 1997;71(6):345–356. doi: 10.1159/000244435. [DOI] [PubMed] [Google Scholar]
- Zahorodny W., Rom C., Whitney W., Giddens S., Samuel M., Maichuk G., Marshall R. The neonatal withdrawal inventory: a simplified score of newborn withdrawal. J Dev Behav Pediatr. 1998 Apr;19(2):89–93. doi: 10.1097/00004703-199804000-00005. [DOI] [PubMed] [Google Scholar]


