Abstract
Aims: To examine derived indices of ß cell function, peripheral insulin sensitivity, and the pancreatic response to intravenous glucose loading in children with a previous history of transient neonatal diabetes currently in remission, repeated after a period of two or more years.
Methods: The standard intravenous glucose tolerance test (IVGTT) was used to measure the first phase insulin response (FPIR) cumulatively at one and three minutes. In addition, fasting insulin and glucose values were used to estimate insulinogenic indices (ß cell function) and QUICKI (insulin sensitivity).
Patients: Six patients with known previous transient neonatal diabetes currently in remission with no exogenous insulin requirement were tested. Control data from 15 children of a similar age were available for derived fasting indices of ß cell functional capacity and insulin sensitivity.
Results: One child had a subnormal insulin secretory response to intravenous glucose that remained abnormal two and four years later. The other children had relatively normal or entirely normal responses over two years. Measures of ß cell function and insulin sensitivity in the fasting state showed comparable results to those obtained from normal controls.
Conclusions: Most children with transient neonatal diabetes in remission have no evidence of ß cell dysfunction or insulin resistance in the fasting state, although they might have been expected to show subtle defects given the tendency to relapse in adolescence. Measures of insulin response to intravenous glucose loading are often normal but suggest future recurrence if profoundly abnormal.
Full Text
The Full Text of this article is available as a PDF (62.1 KB).
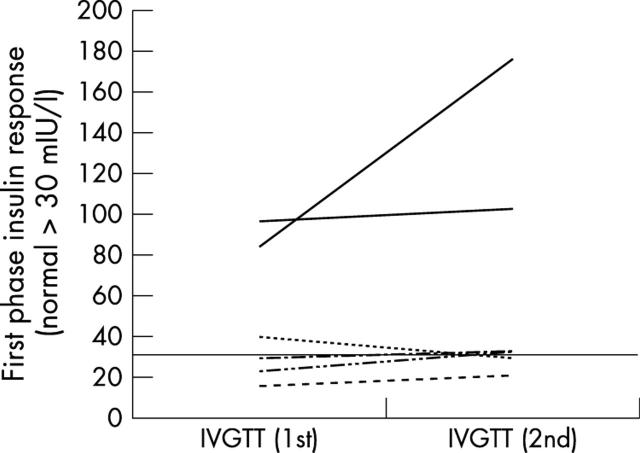
Figure 1.
First phase insulin response (normal > 30 mIU/l) to intravenous glucose in the six cases of transient diabetes mellitus in remission over an interval of two years. The horizontal line represents the 5th centile from historical controls without diabetes.13 The dashed line shows the response of the child who developed impaired glucose tolerance on the oral glucose tolerance test. IGTT, Intravenous glucose tolerance test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingley P. J., Colman P., Eisenbarth G. S., Jackson R. A., McCulloch D. K., Riley W. J., Gale E. A. Standardization of IVGTT to predict IDDM. Diabetes Care. 1992 Oct;15(10):1313–1316. doi: 10.2337/diacare.15.10.1313. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol Metab. 2000 Nov;11(9):375–378. doi: 10.1016/s1043-2760(00)00305-2. [DOI] [PubMed] [Google Scholar]
- Cavé H., Polak M., Drunat S., Denamur E., Czernichow P. Refinement of the 6q chromosomal region implicated in transient neonatal diabetes. Diabetes. 2000 Jan;49(1):108–113. doi: 10.2337/diabetes.49.1.108. [DOI] [PubMed] [Google Scholar]
- Chevenne D., Leger J., Levy-Marchal C., Noel M., Collin D., Czernichow P., Porquet D. Proinsulin and specific insulin responses to an oral glucose tolerance test in a healthy population. Diabetes Metab. 1998 Jun;24(3):260–261. [PubMed] [Google Scholar]
- Ciani E., Hoffmann A., Schmidt P., Journot L., Spengler D. Induction of the PAC1-R (PACAP-type I receptor) gene by p53 and Zac. Brain Res Mol Brain Res. 1999 Jun 8;69(2):290–294. doi: 10.1016/s0169-328x(99)00116-3. [DOI] [PubMed] [Google Scholar]
- Gardner R. J., Mackay D. J., Mungall A. J., Polychronakos C., Siebert R., Shield J. P., Temple I. K., Robinson D. O. An imprinted locus associated with transient neonatal diabetes mellitus. Hum Mol Genet. 2000 Mar 1;9(4):589–596. doi: 10.1093/hmg/9.4.589. [DOI] [PubMed] [Google Scholar]
- Metz Chantal, Cavé Hélène, Bertrand Anne Marie, Deffert Christine, Gueguen-Giroux Béatrice, Czernichow Paul, Polak Michel, NDM French Study Group. Neonatal diabetes mellitus Neonatal diabetes mellitus: chromosomal analysis in transient and permanent cases. J Pediatr. 2002 Oct;141(4):483–489. doi: 10.1067/mpd.2002.127089. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Deschamps I., Chevenne D., Roger M., Mogenet A., Boitard C. Relationship between first-phase insulin secretion and age, HLA, islet cell antibody status, and development of type I diabetes in 220 juvenile first-degree relatives of diabetic patients. Diabetes Care. 1991 Aug;14(8):718–723. doi: 10.2337/diacare.14.8.718. [DOI] [PubMed] [Google Scholar]
- Scaglia L., Smith F. E., Bonner-Weir S. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology. 1995 Dec;136(12):5461–5468. doi: 10.1210/endo.136.12.7588296. [DOI] [PubMed] [Google Scholar]
- Schiff D., Colle E., Stern L. Metabolic and growth patterns in transient neonatal diabetes. N Engl J Med. 1972 Jul 20;287(3):119–122. doi: 10.1056/NEJM197207202870304. [DOI] [PubMed] [Google Scholar]
- Shield J. P., Gardner R. J., Wadsworth E. J., Whiteford M. L., James R. S., Robinson D. O., Baum J. D., Temple I. K. Aetiopathology and genetic basis of neonatal diabetes. Arch Dis Child Fetal Neonatal Ed. 1997 Jan;76(1):F39–F42. doi: 10.1136/fn.76.1.f39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield J. P., Howell W. M., Temple I. K. Neonatal diabetes. Diabetes Care. 1997 Jun;20(6):1045–1046. doi: 10.2337/diacare.20.6.1045c. [DOI] [PubMed] [Google Scholar]
- Shield J. P. Neonatal diabetes: new insights into aetiology and implications. Horm Res. 2000;53 (Suppl 1):7–11. doi: 10.1159/000053198. [DOI] [PubMed] [Google Scholar]
- Sobey W. J., Beer S. F., Carrington C. A., Clark P. M., Frank B. H., Gray I. P., Luzio S. D., Owens D. R., Schneider A. E., Siddle K. Sensitive and specific two-site immunoradiometric assays for human insulin, proinsulin, 65-66 split and 32-33 split proinsulins. Biochem J. 1989 Jun 1;260(2):535–541. doi: 10.1042/bj2600535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D., Villalba M., Hoffmann A., Pantaloni C., Houssami S., Bockaert J., Journot L. Regulation of apoptosis and cell cycle arrest by Zac1, a novel zinc finger protein expressed in the pituitary gland and the brain. EMBO J. 1997 May 15;16(10):2814–2825. doi: 10.1093/emboj/16.10.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple I. K., Gardner R. J., Robinson D. O., Kibirige M. S., Ferguson A. W., Baum J. D., Barber J. C., James R. S., Shield J. P. Further evidence for an imprinted gene for neonatal diabetes localised to chromosome 6q22-q23. Hum Mol Genet. 1996 Aug;5(8):1117–1121. doi: 10.1093/hmg/5.8.1117. [DOI] [PubMed] [Google Scholar]
- Temple I. K., James R. S., Crolla J. A., Sitch F. L., Jacobs P. A., Howell W. M., Betts P., Baum J. D., Shield J. P. An imprinted gene(s) for diabetes? Nat Genet. 1995 Feb;9(2):110–112. doi: 10.1038/ng0295-110. [DOI] [PubMed] [Google Scholar]
- Uwaifo Gabriel I., Fallon Erica M., Chin Jeff, Elberg Jane, Parikh Shamik J., Yanovski Jack A. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002 Nov;25(11):2081–2087. doi: 10.2337/diacare.25.11.2081. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N. Pituitary adenylate cyclase activating polypeptide enhances glucose-evoked insulin secretion in the canine pancreas in vivo. JOP. 2001 Sep;2(5):306–316. [PubMed] [Google Scholar]