Abstract
Background: Glucose-6-phosphate dehydrogenase (G6PD) activity is higher in term neonates than in adults. Some studies have suggested that activity may be even higher in preterm infants.
Objectives: To determine if G6PD activity is higher in preterm than term neonates, and whether higher activity would interfere with diagnosis of G6PD deficiency in premature infants.
Methods: G6PD activity was determined in the first 48 hours after delivery in male premature, term, and near term infants. G6PD deficient neonates were separated, and the remaining premature infants compared with healthy, male, G6PD normal, near term and term neonates.
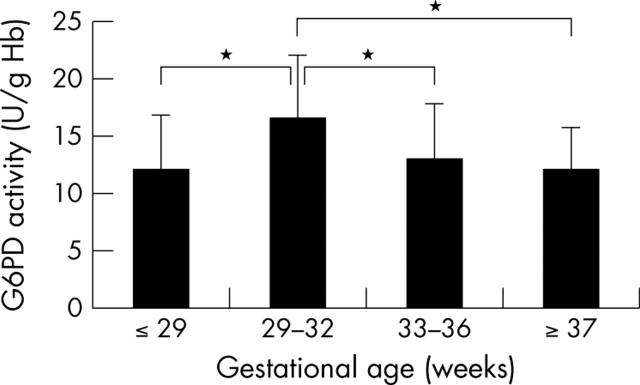
Results: Ninety four premature infants (mean (SD) gestational age 31.9 (3.8) weeks (range 23–36)) were studied. In four, G6PD activity was 0.8–1.8 U/g haemoglobin (Hb), which is clearly in the deficient range with no overlap into the normal range. G6PD activity in the remaining premature infants was significantly higher than in 24 near term and term neonates (gestational age ⩾ 37 weeks) (14.2 (4.6) v 12.0 (3.8) U/g Hb). Further analysis showed that significance was limited to those born between 29 and 32 weeks gestation, in which group G6PD activity was significantly higher than in those born before 29 weeks gestation, at 33–36 weeks gestation, and ⩾ 37 weeks gestation.
Conclusions: G6PD activity is higher in premature infants born between 29 and 32 weeks gestation than in term neonates. This did not interfere with diagnosis of G6PD deficiency.
Full Text
The Full Text of this article is available as a PDF (62.2 KB).
Figure 1.
Glucose-6-phosphate dehydrogenase (G6PD) activity (mean (SD)) for the three subgroups of premature infants and the term and near term neonates. *p < 0.001.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E. G6PD deficiency. Blood. 1994 Dec 1;84(11):3613–3636. [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Herz F., Kaplan E., Scheye E. S. A longitudinal study of red cell enzymes in infants of low birth weight. Z Kinderheilkd. 1975 Sep 11;120(3):217–221. doi: 10.1007/BF00439011. [DOI] [PubMed] [Google Scholar]
- Johnson Lois H., Bhutani Vinod K., Brown Audrey K. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr. 2002 Apr;140(4):396–403. doi: 10.1067/mpd.2002.123098. [DOI] [PubMed] [Google Scholar]
- Kaplan M., Hammerman C. Severe neonatal hyperbilirubinemia. A potential complication of glucose-6-phosphate dehydrogenase deficiency. Clin Perinatol. 1998 Sep;25(3):575-90, viii. [PubMed] [Google Scholar]
- Kaplan M., Leiter C., Hammerman C., Rudensky B. Enzymatic activity in glucose-6-phosphate dehydrogenase-normal and -deficient neonates measured with a commercial kit. Clin Chem. 1995 Nov;41(11):1665–1667. [PubMed] [Google Scholar]
- Kaplan M., Renbaum P., Levy-Lahad E., Hammerman C., Lahad A., Beutler E. Gilbert syndrome and glucose-6-phosphate dehydrogenase deficiency: a dose-dependent genetic interaction crucial to neonatal hyperbilirubinemia. Proc Natl Acad Sci U S A. 1997 Oct 28;94(22):12128–12132. doi: 10.1073/pnas.94.22.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad P. N., Valentine W. N., Paglia D. E. Enzymatic activities and glutathione content of erythrocytes in the newborn: comparison with red cells of older normal subjects and those with comparable reticulocytosis. Acta Haematol. 1972;48(4):193–201. doi: 10.1159/000208458. [DOI] [PubMed] [Google Scholar]
- MARKS P. A., GROSS R. T. Erythrocyte glucose-6-phosphate dehydrogenase deficiency: evidence of differences between Negroes and Caucasians with respect to this genetically determined trait. J Clin Invest. 1959 Dec;38:2253–2262. doi: 10.1172/JCI104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Fielek S., Wurzinger K. H. Characteristics of enzymes of erythrocytes from newborn infants and adults: activity, thermostability, and electrophoretic profile as a function of cell age. Am J Hematol. 1981 Sep;11(2):125–136. doi: 10.1002/ajh.2830110203. [DOI] [PubMed] [Google Scholar]
- Oppenheim A., Jury C. L., Rund D., Vulliamy T. J., Luzzatto L. G6PD Mediterranean accounts for the high prevalence of G6PD deficiency in Kurdish Jews. Hum Genet. 1993 Apr;91(3):293–294. doi: 10.1007/BF00218277. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Smith C. Red cell metabolism in the premature infant. 3. Apparent inappropriate glucose consumption for cell age. Pediatrics. 1968 Feb;41(2):473–482. [PubMed] [Google Scholar]
- STEWART A. G., BIRKBECK J. A. The activities of lactate dehydrogenase, transaminase, and glucose-6-phosphate dehydrogenase in the erythrocytes and plasma of newborn infants. J Pediatr. 1962 Sep;61:395–404. doi: 10.1016/s0022-3476(62)80370-9. [DOI] [PubMed] [Google Scholar]
- Travis S. F., Kumar S. P., Paez P. C., Delivoria-Papadopoulos M. Red cell metabolic alterations in postnatal life in term infants: glycolytic enzymes and glucose-6-phosphate dehydrogenase. Pediatr Res. 1980 Dec;14(12):1349–1352. doi: 10.1203/00006450-198012000-00016. [DOI] [PubMed] [Google Scholar]
- Valaes T. Severe neonatal jaundice associated with glucose-6-phosphate dehydrogenase deficiency: pathogenesis and global epidemiology. Acta Paediatr Suppl. 1994 Mar;394:58–76. doi: 10.1111/j.1651-2227.1994.tb13216.x. [DOI] [PubMed] [Google Scholar]