Abstract
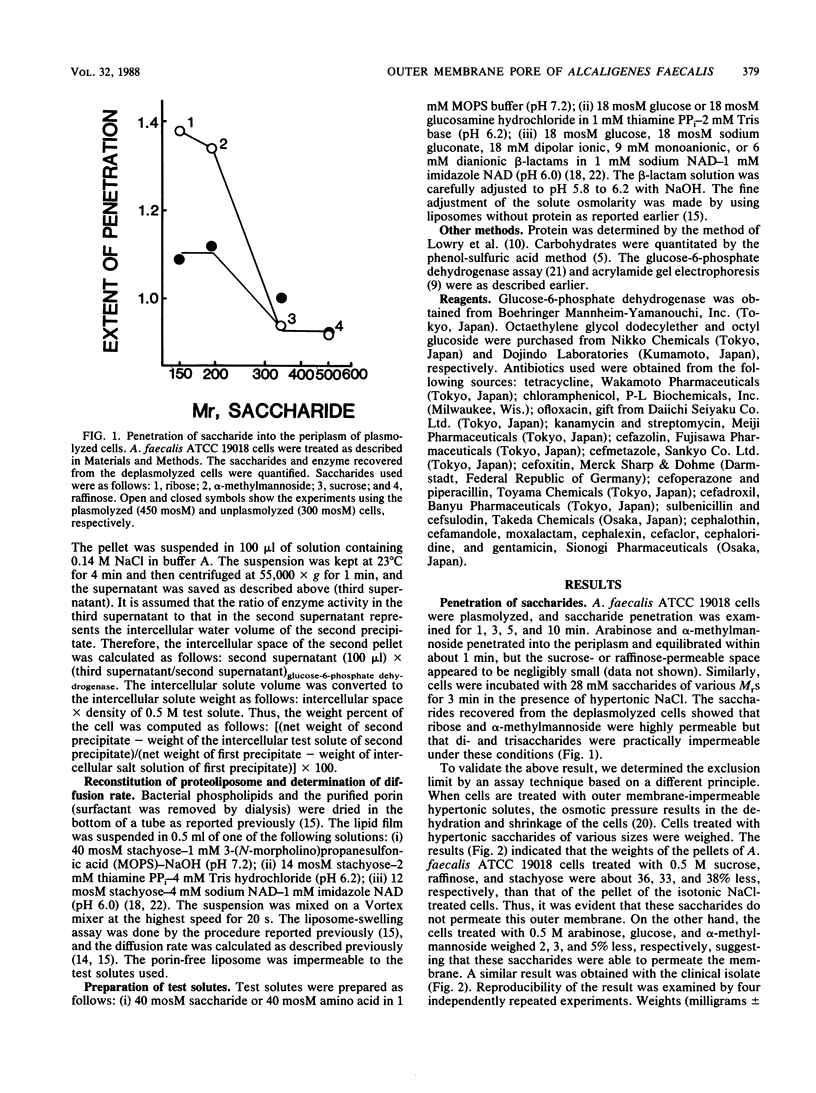
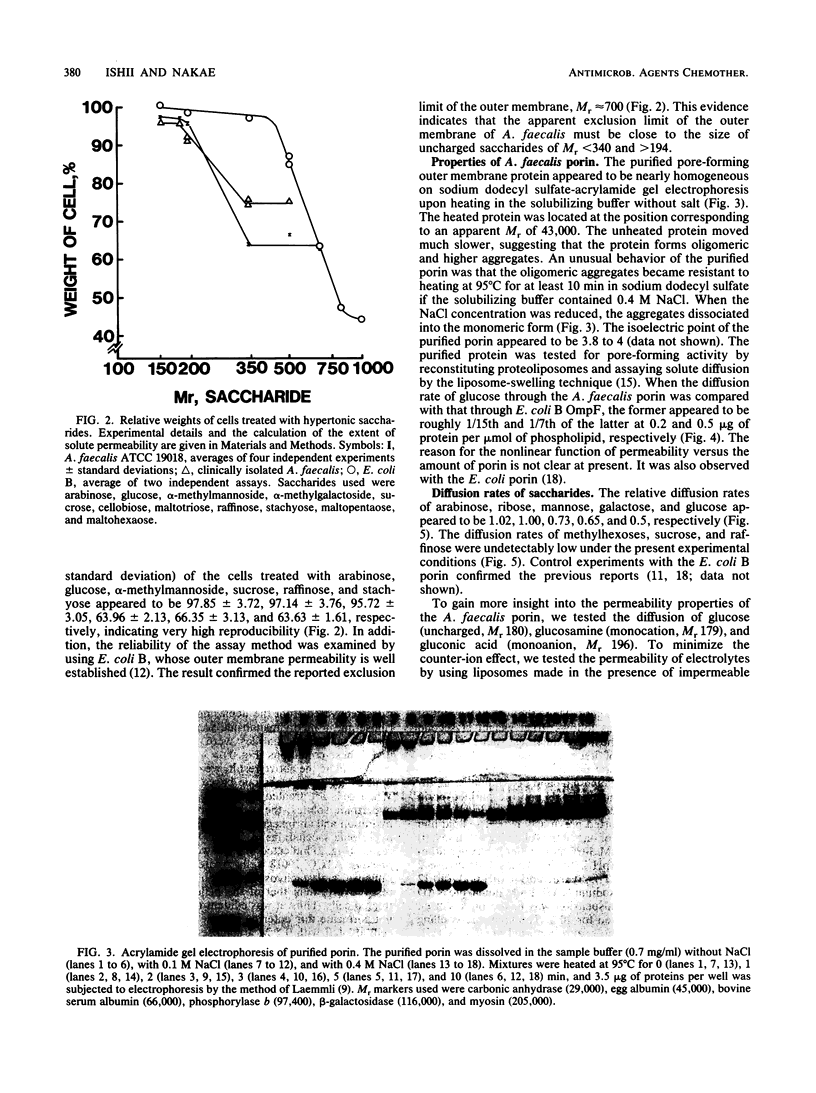
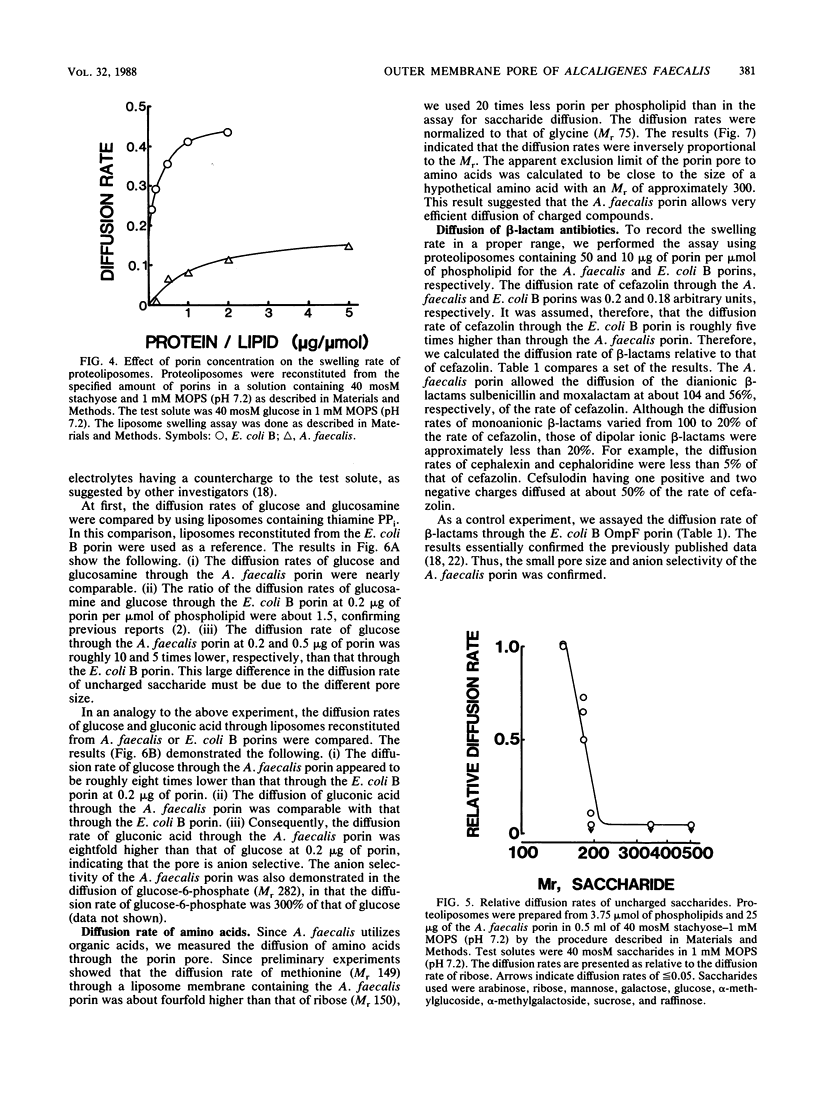
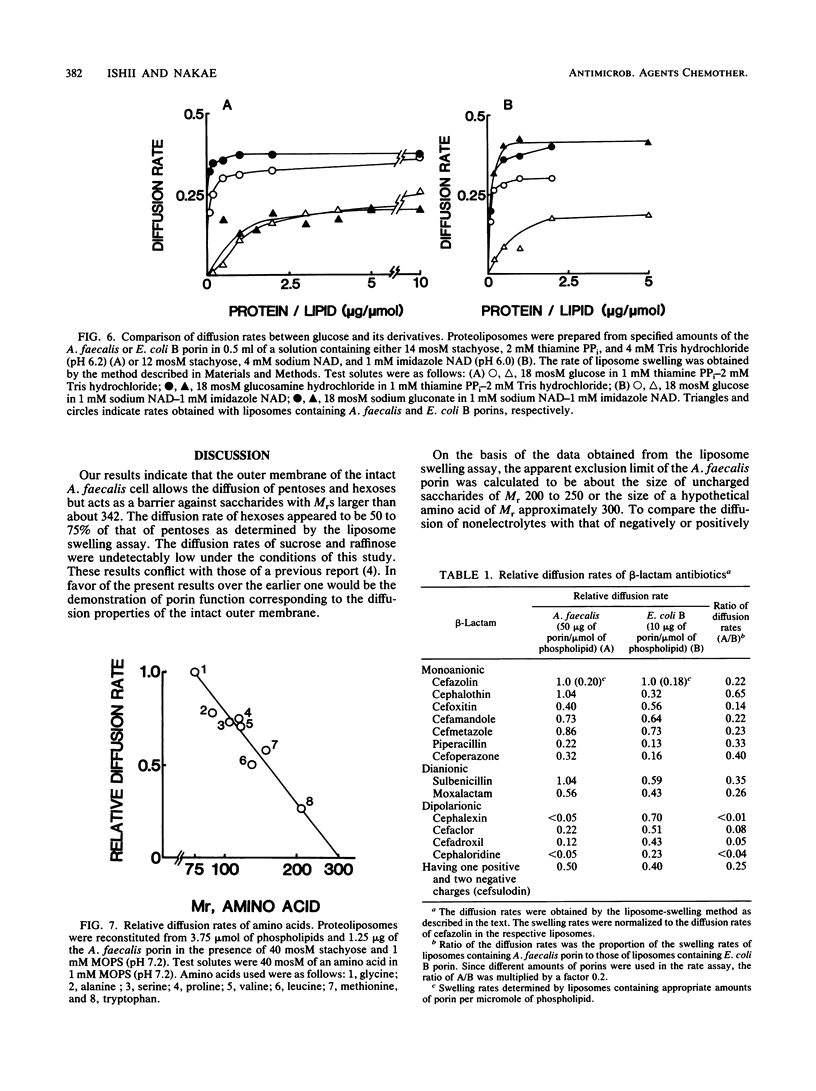
The diffusion pore of the outer membrane of Alcaligenes faecalis was shown to be substantially smaller than the Escherichia coli porin pore. In experiments with intact cells, pentoses and hexoses penetrated into the NaCl-expanded periplasm, whereas saccharides of Mr greater than 342 did not. Cells treated with 0.5 M saccharides of Mr greater than 342 weighed 33 to 38% less than cells treated with isotonic solution, suggesting that these saccharides do not permeate through the outer membrane. The diffusion rates of various solutes through the liposome membranes reconstituted from the Mr-43,000 outer membrane protein showed the following characteristics. (i) The relative diffusion rates of pentoses, hexoses, and methylhexoses appeared to be about 1.0, 0.6, and negligibly small, respectively. (ii) The diffusion rate of glucose appeared to be about 1/10th that with the E. coli B porin. (iii) The diffusion rate of gluconic acid was five to seven times higher than that of glucose. (iv) The diffusion rates of beta-lactam antibiotics appeared to be 40 to less than 10% of those with the E. coli B porin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Schmid A., Hancock R. E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985 May;162(2):722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS M., GILLES K., HAMILTON J. K., REBERS P. A., SMITH F. A colorimetric method for the determination of sugars. Nature. 1951 Jul 28;168(4265):167–167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Barnishan J. In vitro susceptibilities of nonfermentative gram-negative bacilli other than Pseudomonas aeruginosa to 32 antimicrobial agents. Rev Infect Dis. 1980 Nov-Dec;2(6):841–853. doi: 10.1093/clinids/2.6.841. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakae R., Nakae T. Diffusion of aminoglycoside antibiotics across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 1982 Oct;22(4):554–559. doi: 10.1128/aac.22.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Ishii J., Ferenci T. The role of the maltodextrin-binding site in determining the transport properties of the LamB protein. J Biol Chem. 1986 Jan 15;261(2):622–626. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nakae T. Outer-membrane permeability of bacteria. Crit Rev Microbiol. 1986;13(1):1–62. doi: 10.3109/10408418609108734. [DOI] [PubMed] [Google Scholar]
- Nayler J. H. Resistance to beta-lactams in gram-negative bacteria: relative contributions of beta-lactamase and permeability limitations. J Antimicrob Chemother. 1987 Jun;19(6):713–732. doi: 10.1093/jac/19.6.713. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga H., Tokunaga M., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 1. Chemical analysis. Eur J Biochem. 1979 Apr;95(3):433–439. doi: 10.1111/j.1432-1033.1979.tb12982.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama H., Akatsuka A., Nakae T. The outer membrane of Pseudomonas aeruginosa is a barrier against the penetration of disaccharides. Biochem Biophys Res Commun. 1986 Jan 14;134(1):106–112. doi: 10.1016/0006-291x(86)90533-4. [DOI] [PubMed] [Google Scholar]
- Yoneyama H., Nakae T. A small diffusion pore in the outer membrane of Pseudomonas aeruginosa. Eur J Biochem. 1986 May 15;157(1):33–38. doi: 10.1111/j.1432-1033.1986.tb09634.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]




