Abstract
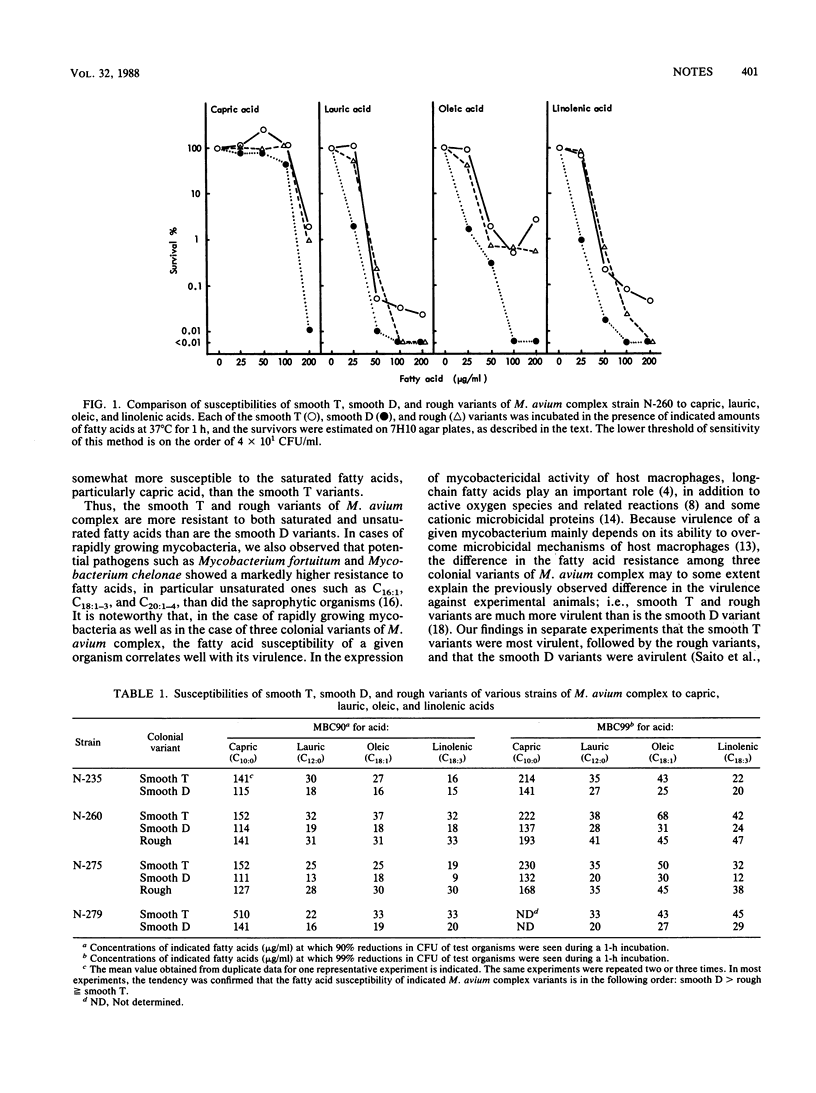
Three different colonial variants of Mycobacterium avium complex were studied for their susceptibilities to capric, lauric, oleic, and linolenic acids. Smooth T variants with transparent and irregularly shaped colonies were much more resistant to all the fatty acids than were the smooth D variants with opaque and dome-shaped colonies. Rough variants with granular and irregularly shaped colonies showed nearly the same susceptibility to the fatty acids as did the smooth T variants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsworth G. R., Kochan I. Secretion of antimycobacterial fatty acids by normal and activated macrophages. Infect Immun. 1978 Jan;19(1):170–177. doi: 10.1128/iai.19.1.170-177.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J., Gordon N., Kajioka R. Permeability barrier to rifampin in mycobacteria. Antimicrob Agents Chemother. 1977 May;11(5):773–779. doi: 10.1128/aac.11.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai K., Kondo E. Antibacterial and cytotoxic aspects of long-chain fatty acids as cell surface events: selected topics. Jpn J Med Sci Biol. 1979 Jun;32(3):135–174. doi: 10.7883/yoken1952.32.135. [DOI] [PubMed] [Google Scholar]
- Kanai K., Kondo E. Phospholipase A2-induced antimycobacterial activity in the membrane fraction obtained from peritoneal exudate cells of guinea pigs. Jpn J Med Sci Biol. 1980 Apr;33(2):87–101. doi: 10.7883/yoken1952.33.87. [DOI] [PubMed] [Google Scholar]
- Kondo E., Kanai K. The relationship between the chemical structure of fatty acids and their mycobactericidal activity. Jpn J Med Sci Biol. 1977 Aug;30(4):171–178. doi: 10.7883/yoken1952.30.171. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B. How macrophages kill tubercle bacilli. J Med Microbiol. 1983 Feb;16(1):1–12. doi: 10.1099/00222615-16-1-1. [DOI] [PubMed] [Google Scholar]
- McCarthy C. Spontaneous and Induced Mutation in Mycobacterium avium. Infect Immun. 1970 Sep;2(3):223–228. doi: 10.1128/iai.2.3.223-228.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Fukunaga M., Taniguchi H. Plasmid deoxyribonucleic acid and translucent-to-opaque variation in Mycobacterium intracellulare 103. J Bacteriol. 1981 May;146(2):656–659. doi: 10.1128/jb.146.2.656-659.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Udou T., Yamada T. Mechanism of antibiotic resistance in Mycobacterium intracellulare. Microbiol Immunol. 1983;27(5):425–431. doi: 10.1111/j.1348-0421.1983.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Delafield J., Martinez R. J., Lehrer R. I. Microbicidal cationic proteins in rabbit alveolar macrophages: a potential host defense mechanism. Infect Immun. 1980 Oct;30(1):180–192. doi: 10.1128/iai.30.1.180-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Yoneyama T. Growth of group IV mycobacteria on medium containing various saturated and unsaturated fatty acids. Antimicrob Agents Chemother. 1984 Aug;26(2):164–169. doi: 10.1128/aac.26.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo A., Della Bruna C., Marsili L., Morvillo E., Pasqualucci C. R., Schioppacassi G., Ungheri D. Biological activity of a new class of rifamycins. Spiro-piperidyl-rifamycins. J Antibiot (Tokyo) 1980 Oct;33(10):1193–1198. doi: 10.7164/antibiotics.33.1193. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):516–524. doi: 10.1128/jb.111.2.516-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J Bacteriol. 1973 Sep;115(3):869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley C. L., David H. L. Effect of temperature on the rate of the transparent to opaque colony type transition in Mycobacterium avium. Antimicrob Agents Chemother. 1976 Jan;9(1):113–119. doi: 10.1128/aac.9.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]



