Abstract
A transgenic (Tg) mouse expressing human IL-15 was generated to define the role of IL-15 in the normal immune response. Overexpression of IL-15 resulted in an increase of NK, CD44hiCD8 memory T cells, and γδ T cells. Additionally, we observed the emergence of a novel type of NK-T cells with CD8αα′ expression. Due to the expansion and activation of NK cells, the IL-15Tg mouse showed enhanced innate immunity. In adaptive T cell immunity, the roles of IL-15 contrasted with those of IL-2. IL-15 inhibited IL-2-induced T cell death, which plays a role in the maintenance of peripheral self-tolerance. IL-15 thus seems to contribute to enhanced immune memory by selectively propagating memory T cells and by blocking T cell death mediated by IL-2.
The challenge of the immune system is to generate an effective immune response including the generation of effective memory cells to external pathogens, yet to be tolerant to self. For this purpose, both central tolerance in the thymus and peripheral tolerance play important roles. Additionally, different roles are assigned to innate and adaptive immunity to achieve this goal. Interleukin (IL)-2 and IL-15 (1–3), among many cytokines, are major participants in this regard. In T/NK cells, IL-15 shares receptor components with IL-2, thus the biological activities of these two lymphokines overlap (2–4). Many biological functions have been proposed for IL-15 from in vitro studies (reviewed in ref. 5). However, IL-15 is produced by nonlymphoid cells including monocytes (6), dendritic cells (7, 8), and bone marrow stromal cells (9–11), whereas IL-2 is produced exclusively by activated T lymphocytes. In addition, the distribution of the ligand-specific receptor component IL-2Rα/CD25 is different from that of IL-15Rα (4). Collectively, these data suggested that IL-2 and IL-15 might exert distinct functions in vivo. Indeed, we have previously demonstrated that only IL-15, and not IL-2, activates mast cell proliferation through the use of a novel receptor/signaling system (12, 13). Even in T/NK cells where the conventional IL-2/15Rβ and γc are used for IL-15 signaling, accumulating evidence suggests more differences than similarities between IL-2 and IL-15 functions. For example, mice deficient in IL-2Rα or IL-15Rα displayed sharp contrasts in their phenotypes. In the IL-2Rα−/− mouse, a polyclonal T cell expansion was observed associated with autoimmune diseases, but the animal had normal numbers of NK cells (14). In contrast, NK, NK-T, CD8 T, and γδ T cells were deficient in the IL-15Rα−/− mouse, without autoreactive T cell propagation (15). Similarly, IL-2−/− (16) and IL-15−/− (17) mice showed marked differences in their phenotypic characteristics. These pieces of data support the hypothesis that IL-2 and IL-15 are molecules with distinct functions (18).
As one of the means to address IL-15 functions in vivo, we generated a transgenic mouse expressing human IL-15. Analyses of the IL-15Tg mouse allowed us to reassess IL-15 functions in the persistent presence of this cytokine. Indeed, we recapitulated the importance and involvement of IL-15 in the development of NK cells and memory phenotype CD8 T cells (19). We also observed that innate immunity was more efficient against viral infections in the IL-15Tg mouse. Unexpectedly, the adaptive immunity of the IL-15Tg mouse was distinct in that the IL-2-induced activation-induced cell death (AICD), a major mechanism underlying peripheral self-tolerance (20), was inhibited by the presence of IL-15. Collectively, these pieces of evidence support the emerging notion that IL-2 and IL-15 must be balanced to achieve appropriate cellular immunity during immune responses (21).
Materials and Methods
Generation of a Transgenic Mouse Expressing Human IL-15.
Complementary DNAs encoding the bovine preprolactin signal peptide (SP), human IL-15 mature coding sequence, human elongation factor 1α promoter (22), and simian virus 40 poly(A) signal were amplified by PCR. The cDNAs were then fused to generate an expression construct, which was microinjected into fertilized eggs of C57BL/6 mice to obtain several founder mice. Hemizygous Tg mice were generated by mating founder mice with wild-type (wt) C57BL/6 mice.
Cells and Cytokine ELISA.
Culture cell lines used in this study were purchased from American Type Culture Collection. Quantitation of IL-15 using murine CTLL-2 cell line is described elsewhere (13). For cytokine ELISA, the Quantikine kits from R & D Systems were used.
Antibodies and Reagents.
Monoclonal antibodies used for flow cytometry (FCM) analyses were purchased from PharMingen. Cellular fluorescence was captured and analyzed by using FACSort (Becton Dickinson). The anti-asialo GM1 antibody used to delete NK cells was purchased from Wako Pure Chemical (Osaka). FCM data represent at least three independent experiments.
Cytotoxicity Assay.
The experimental procedure followed that of the JAM assay (23).
RNase Protection Assay.
Murine glyceraldehyde-3-phosphate dehydrogenase, IL-4, and γ-IFN cDNAs were generated by PCR and subcloned into the pCR2.1 vector (Invitrogen) to prepare the 32P-cRNA probe using in vitro transcription (Ambion, Austin, TX). Five micrograms of RNA was hybridized with the 32P-cRNA overnight at 65°C in 10 μl and then digested by an RNase A/T1 mixture (PharMingen). The resultant RNA–RNA duplex was resolved by a 6% denaturing polyacrylamide gel.
BrdUrd Incorporation Assay.
Mice were given drinking water containing 0.8 g/ml BrdUrd (Sigma) for 4 days. Cells were fixed with 70% ethanol and stained by an anti-BrdUrd antibody (Becton Dickinson).
Virus Infection Experiments.
Briefly, 2 × 108 plaque-forming units of wild-type Syeth vaccinia virus (a kind gift from Jeffrey Schlom, National Cancer Institute) were injected (i.v.) into five Tg and five age-matched wt mice. At the indicated time postinfection, blood was collected and analyzed by a standard CV-1 plaque assay.
Activation-Induced Cell Death.
Splenic CD4 T cells were purified by using a negative selection (MACS system, Miltenyi Biotec, Auburn, CA). Purified CD4 T cells (>95% in purity) were stimulated by a plate-coated anti-CD3ɛ antibody (145-2C11, PharMingen) in the presence of 5 μg/ml soluble anti-CD28 antibody (clone 37.51, PharMingen) for 48 h, then transferred to the cytokine culture with 1 nM IL-2 for 48 additional h. Cells were then finally restimulated with plate-coated anti-CD3/CD28 antibodies to induce AICD. Apoptotic cells were defined by propidium iodide/Annexin V-GFP (CLONTECH) staining and then analyzed by FCM.
Results
Expression of the Human IL-15 Transgene in the IL-15Tg Mouse.
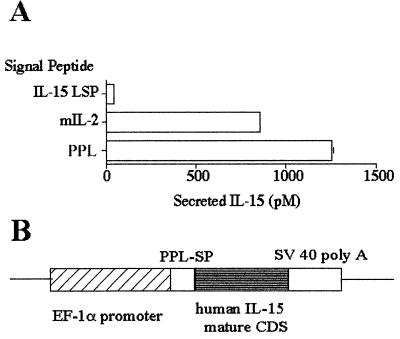
We reported that the expression of IL-15 protein is negatively regulated posttranscriptionally by inefficient translation and intracellular trafficking (12, 24). Therefore, experiments were first carried out to design a transgene construct that would enable an optimal secretion of IL-15. Two elements were examined. One was the choice of the SP and the other concerned that of the mature coding sequence. We reported that two isoforms of IL-15 SPs exist and that only the IL-15 with the long signal peptide (LSP) (48 aa) is secreted (25). However, this LSP was not very efficient in secreting IL-15, as substitution of the LSP by the IL-2 SP increased IL-15 secretion (24). We tested IL-2 and bovine preprolactin SPs and chose the preprolactin–SP, as this gave the best secretion when examined in the COS-7 expression assay (Fig. 1A). Previous reports support the notion that NK cell induction and T cell activation in mice are successfully achieved by human IL-15 (9, 19). In addition, limited resources are available for studying murine IL-15 functions. Therefore, we decided to express human IL-15 in the mouse. The construct design used is shown in Fig. 1B.
Figure 1.
Generation of the IL-15 transgenic mouse. (A) Ten micrograms of construct bearing the IL-15 SP (long signal peptide, LSP), IL-2 SP, or preprolactin (PPL) SP fused with human IL-15 cDNA (coding the mature peptide) under the human EF-1α promoter was transfected into 4 × 106 COS cells by electroporation (250 V/950 μF). The supernatants were analyzed by a CTLL-2 assay at 48 h posttransfection. (B) Schematic design of the construct used to generate the IL-15Tg mouse.
After establishing seven founders that showed transgene integration, F1 mice from each founder were tested for the serum levels of human IL-15 by using a commercial ELISA kit. We observed detectable levels of human IL-15 from three strains: K2, B1, and J2. The K2 strain expressed the highest amount of IL-15 (650–800 pg/ml, 40–65 pM), and B1 mice had 150–200 pg/ml IL-15 in their serum. For most studies shown below, we used hemizygous mice from the K2 and B1 strains to confirm that any obtained phenotype was a consequence of the overexpression of IL-15 and not due to the disruption of other cellular gene functions by the transgene insertion.
The IL-15Tg Mouse Has Increased Numbers of NK Cells.
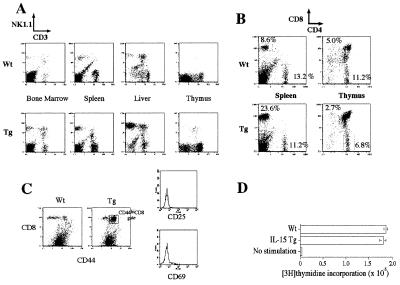
We next determined whether the overexpression of human IL-15 in the mouse led to phenotypic changes in the lymphoid compartment. Numbers of previous reports demonstrated a pivotal role for IL-15 in the development and maintenance of NK (9, 10, 15, 26, 27), NK-T (9, 10, 15), and CD8+ T cells (15, 19), as well as γδ+ intraepithelial T cells (15, 28). Indeed, we observed an increase of NK1+ CD3− cells (Fig. 2A) in the bone marrow (from 0.8% to 7.6%), spleen (from 3.4% to 8.0%), and liver (from 16.1% to 33.3%). There was a correlation between the serum IL-15 level and the increase of NK cells when we compared mice from K2 and B1 strains (summarized in Table 1). Interestingly, we did not observe the increase of NK cells in the thymus.
Figure 2.
Expansion of select lymphoid cells in the IL-15Tg mouse. (A) Analyses of cells from various tissues of wt and IL-15Tg mice. y axis, phosphatidylethanolamine-conjugated NK1.1 antibody; x axis, FITC-conjugated anti-CD3ɛ antibody. (B) Preferential increase of CD8 T cells in the IL-15Tg mouse. (C) Increase of CD44hiCD8 T cells in the IL-15Tg mouse. Memory CD8 T cells were defined by CD44/CD8 double staining (Left). Transgenic CD8 cells show high CD44 expression. CD44hiCD8 cells were further gated for the expression of the early T cell activation markers (FITC–anti-CD69 or anti-CD25) and demonstrated the lack of the expression of these antigens. (D) Normal response of T cells from the IL-15Tg mouse after anti-CD3 stimulation. Lymphocytes from wt and IL-15Tg spleens were incubated with a plate-coated anti-CD3 antibody for 48 h, then pulsed with 1 μCi per well of [3H]thymidine for 6 additional h.
Table 1.
Increase of NK, NK-T, CD8, and γδ T cells in two strains (B1 and K2) of the IL-15 transgenic mouse
| NK, % in lymphocytes
|
NK-T, % in
lymphocytes
|
CD4, % in lymphocytes
|
CD8,
% in CD3 T cells
|
γδ dendritic epidermal T cells
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt | B1 | K2 | Wt | B1 | K2 | Wt | B1 | K2 | Wt | B1 | K2 | Wt | K2 | |
| Bone marrow | 0.8 | NT | 7.6 | 0.7 | NT | 4.7 | NT | NT | NT | NT | NT | NT | NT | NT |
| Liver | 16.1 | 15.5 | 33.3 | 11.3 | 20.9 | 30.7 | NT | NT | NT | 31.7 | NT | 80.1 | NT | NT |
| Spleen | 3.4 | 4.1 | 8.0 | 2.7 | 1.5 | 4.6 | 13.2 | 12.6 | 11.2 | 33.8 | 41.2 | 74.2 | NT | NT |
| Thymus | 0 | NT | 0.2 | 0.5 | NT | 1.6 | 11.2 | NT | 6.8 | 30.8 | NT | 28.4 | NT | NT |
| Skin | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | 1,080 ± 173 (mm2) | 10,122 ± 661 (mm2) |
NT, Not tested.
Increase in the Proportion of CD8 But Not CD4 T Cells in the IL-15Tg Mouse.
We next examined T cell subsets in the IL-15Tg mouse. As shown in Fig. 2B, we observed a preferential expansion of CD8 T cells in the IL-15Tg mouse. The number of CD4 cells from the IL-15Tg spleen was close to that of wt mice, indicating that the CD8 population was selectively propagated in the IL-15Tg mice. Again, this preferential expansion of CD8 T cells was not observed in the thymus, suggesting that these IL-15 functions are associated with cells in the peripheral lymphoid compartment. Zhang et al. (19) have demonstrated that injection of IL-15 protein into mice facilitated a propagation of CD8 T cells with memory phenotypes. To examine whether such an expansion occurred under the persistent presence of IL-15, CD8 cells from the IL-15Tg mouse were stained and analyzed for their expression of surface markers associated with memory T cells. Fig. 2C indicates the skewed high expression of the CD44 antigen on the CD8+ cells in the IL-15Tg mouse. These CD44hiCD8 cells showed a CD45RAlow (data not shown) CD25−CD69− phenotype, thus bearing markers for functional memory–phenotype T cells.
To examine whether T cells from the IL-15Tg mouse showed any functional defects in their response to antigen-like stimulation, the response of splenic lymphocytes to anti-CD3 stimulation was monitored by the incorporation of [3H]thymidine. As shown in Fig. 2D, no difference was observed between lymphocytes from wt and IL-15Tg mice, suggesting that T cells from the IL-15Tg mouse are not spontaneously activated and that they retain the capacity to respond to primary antigen stimulation.
Increase of CD8+ NK-T But Not CD4+ NK-T Cells in the IL-15Tg Mouse.
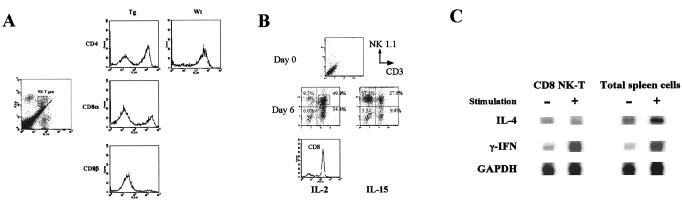
In the IL-15Tg mouse, we observed the propagation of NK1+CD3+ cells. However, a three-color FCM analysis revealed that these were not the typical CD4+ NK-T cells as originally described by Bendelac et al. (29). As shown in Fig. 2A, cells expressing both the NK and CD3 antigens were increased slightly in the bone marrow and spleen. When the CD3+NK1+ cells were gated for further analysis for CD4 or CD8 expression, we observed that a significant fraction (>40%) of NK-T cells in spleen and liver expresses CD8 but not CD4 (Fig. 3A). Such CD8 NK-T cells did not exist in the wt mouse (Fig. 3A). Anti-CD8β antibody failed to stain the same cell population, indicating that these NK-T cells express CD8αα′ rather than CD8αβ (Fig. 3A). Thus, IL-15 seems to induce CD8+ NK-T cells rather than CD4 NK-T cells. Interestingly, an ex vivo culture of wt bone marrow cells with cytokines such as IL-2 and IL-15 led to the propagation of similar CD8+NK-T cells (Fig. 3B). Therefore, IL-2, in addition to IL-15, is capable of inducing such cells, and CD8 NK-T cells are a cytokine-inducible population in this regard. Ballas and Rasmussen (30) demonstrated an induction of similar CD8+ NK-T cells by IL-4. However, IL-4 was not as efficient as IL-2 or IL-15 in our ex vivo culture system in inducing CD8+ NK-T cells. As shown in Fig. 3C, the CD8 NK-T cells did not produce IL-4 upon CD3 stimulation, a hallmark of the CD4 NK-T cells, suggesting that CD4 and CD8 NK-T cells have distinct cellular functions.
Figure 3.
(A) Properties of NK-T cells observed in the IL-15Tg mouse. Lymphocytes were isolated and purified from the liver. (Left) The NK-T population (FL1, FITC–anti-CD3ɛ; FL2, phycoerythrin–anti-NK1.1). Gated cells were further analyzed in the FL3 channel by the indicated antibodies (for CD8β, biotin–αCD8β/Cychrome–Streptavidin were used). As a control, preferential CD4 staining seen with the wt liver NK-T cells is shown. (B) Induction of CD8 NK-T cells from the bone marrow precursors of wt mice. Bone marrow cells were cultured with 10 nM IL-2 or IL-15. At day 6 and following, there was a propagation of NK and NK-T cells. The NK-T cells were further analyzed for their CD8 expression, which was >85% as shown as the histogram. (C) Production of γIFN but not IL-4 by the CD8 NK-T cells after stimulation examined by an RNase protection assay. NK-T cells were purified from a bone marrow culture using negative selection using the anti-CD8β antibody. NK-T cells and total spleen lymphocytes were stimulated by phorbol-myristate acetate (15 ng/ml) and ionomycin (0.75 μM) for 24 h before RNA extraction.
Expansion of γδ Dendritic Epidermal T Cells in the IL-15Tg Mouse.
We next examined the number of γδ T cells in the epidermal layer from the ears of IL-15Tg mice. As shown in Table 1, we observed a 10-fold increase of γδ+ dendritic epidermal T cells in the skin, demonstrating that IL-15 is a growth factor for the γδ dendritic epidermal T cells in vivo.
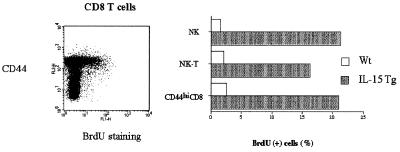
Increase of NK, NK-T, and CD8 T Cells in the IL-15Tg Mouse Is a Consequence of Augmented Cell-Cycle Progression in These Cells.
IL-15 is known to function as an anti-apoptotic factor (31). To determine whether IL-15 had increased select cells in the IL-15Tg mouse by acting as a growth factor or anti-death factor, mice were labeled with BrdUrd in the drinking water for 4 days, and the cells were analyzed for the BrdUrd incorporation. As shown in Fig. 4, NK, CD8 NK-T, and CD44hiCD8 T cells from the IL-15Tg mouse displayed a greater incorporation of BrdUrd than did cells from the wt mouse, indicating that IL-15 indeed drove these cells into cell-cycle progression and thereby led to the propagation of these cells.
Figure 4.
Enhanced BrdUrd incorporation into NK, NK-T, and CD44hiCD8 T cells from the IL-15Tg mouse. Cells were labeled with BrdUrd in vivo for 4 days. Lymphocytes were then stained by antibodies for NK and CD3 antigens, fixed, and stained by an FITC—anti-BrdUrd antibody. (Left) The selective incorporation of BrdUrd into the CD44hiCD8 T cells from the IL-15Tg(K2) mouse. Similar experiments were carried out with NK and NK-T cells, showing similar BrdUrd incorporation.
Augmented Spontaneous Cytotoxicity by IL-15Tg Splenic Lymphocytes.
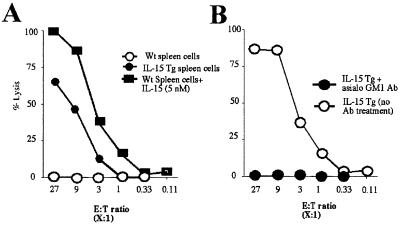
IL-15 augments cytotoxicity of NK cells in vitro (26). We postulated that NK cells from the IL-15Tg mouse might display constitutive cytotoxicity against target cells. To examine this issue, splenic lymphocytes were isolated from wt or IL-15Tg mice, and their spontaneous lytic activity against YAC-1 cells was examined. As shown in Fig. 5A, only splenocytes from the IL-15Tg mouse, but not those from the wt mouse, displayed meaningful lytic activity against YAC-1 cells. The addition of high-dose IL-15 led to the induction of cytotoxicity by wt spleen cells, suggesting that the constitutive lytic activity seen with the Tg splenic cells was directly mediated by the IL-15 produced in the IL-15Tg mouse. We next asked whether or not this spontaneous lysis came solely from NK cells. The intraperitoneal injection of the anti-asialo GM1 antibody did not change the number of CD8+ NK-T or CD8 T cells (data not shown), but the NK cell number dropped almost to zero (8% to 0.3% in the spleen) in the IL-15Tg mouse. After this treatment, splenic lymphocytes from the IL-15Tg no longer showed lytic activity against YAC-1 cells (Fig. 5B), confirming that only classical CD3−NK cells contributed to the cytotoxicity against YAC-1 cells seen with the IL-15Tg mouse. Thus, the activation and propagation of NK cells in the IL-15Tg mouse led to constitutive cytotoxicity in lymphoid organs such as the spleen. This finding supports the importance of IL-15 in regulating the cytotoxicity mediated by NK cells.
Figure 5.
(A) Spontaneous lysis of YAC-1 cells by the IL-15Tg(K2) splenic cells. The effector/target ratio was compensated for based on the percentage of NK cells in the splenic lymphocytes. (B) Depletion of NK cells using the anti-asialo GM1 antibody abrogated the constitutive lytic activity to YAC-1 cells of the IL-15Tg(K2) splenic cells. The injection (i.p.) of the anti-asialo GM1 antibody resulted in rapid disappearance of NK cells from the spleen of the IL-15Tg mouse (8% to 0.3% within 24 h of injection). The NK-depleted splenic lymphocytes were then tested for cytotoxicity.
Augmented Antiviral Immune Response in IL-15Tg Mice.
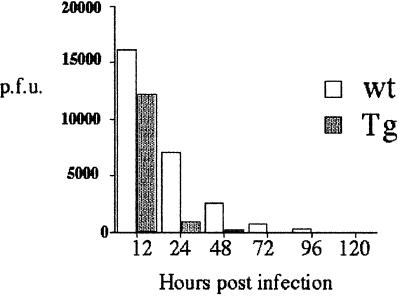
The IL-15Tg mouse displays increased numbers and augmented functions of NK cells, which are involved in various immune responses directed toward eliminating viral infection. Therefore, we next examined whether the IL-15Tg mouse had an increase in innate immunity. To this end, we infected mice with the wt vaccinia virus. As shown in Fig. 6, the viral titer in the serum from IL-15Tg and wt mice was markedly different. Clearance of the virus was much quicker in the IL-15Tg mouse than in the wt control. The observed increase of the NK cell number and activity, as well as the results obtained with the vaccinia infection, demonstrates the augmented innate immunity of the IL-15Tg mouse.
Figure 6.
Augmented viral clearance in the IL-15Tg mouse against the vaccinia virus. 2 × 108 plaque-forming units of the wt vaccinia virus were injected into five wt and IL-15Tg(K2) mice, and their blood was collected at the indicated time points after infection. After removing the plasma, blood cells were lysed by repeated freeze–thaw cycles, and the lysates were incubated with CV-1 cells for 36 h to monitor plaque formation.
Cytokine Production by T Cells from the IL-15Tg Mouse.
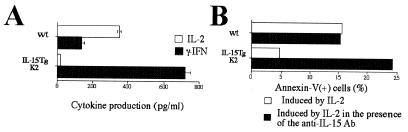
The cytokine network plays an important role in the maintenance of the immune response. The overproduction of IL-15 could theoretically disrupt the cytokine homeostasis in the mouse. To address this issue, CD3 T cells were challenged with an anti-CD3 antibody to examine the production of IL-2 and γ-IFN, two major T cell cytokines. T cells from the IL-15Tg mouse produced higher levels of γ-IFN and lower levels of IL-2 than those from the wt mouse (Fig. 7A). However, such changes might be explained by the change in the ratio between CD4 and CD8, as IL-2 is predominantly produced by CD4 cells whereas CD8 cells produce more γ-IFN than do CD4 cells. However, it is to note that the cytokine environment in the IL-15Tg may be more directed toward γ-IFN than in the wt mouse.
Figure 7.
(A) Reduced IL-2 production from T cells from the IL-15Tg mouse. CD3 T cells from either wt or IL-15Tg(K2) mouse were stimulated by CD3/CD28 antibodies, and cytokine production was measured by specific ELISA. (B) Reduced IL-2-induced AICD by the CD4 T cells purified from the IL-15Tg mouse and the restoration of AICD with the inclusion of an anti-IL-15 neutralizing antibody. CD4 cells (>95% pure) were first activated by CD3/28 antibodies for 48 h and then cultured with 1 nM IL-2 in the presence or absence of the M111 anti-IL-15 Ab (10 μg/ml, Genzyme) for 48 h. Cell death was induced in the third culture period by the treatment with anti-CD3/28 antibodies for 6 h. Dead cells were monitored by staining using the propidium iodide/Annexin V-GFP (CLONTECH). The data represent one of three independent experiments.
Lack of Activation-Induced Cell Death in the IL-15Tg Mouse.
We then turned our efforts to the analysis of adaptive T cell responses in the IL-15Tg mouse. IL-2 is a key cytokine in maintenance of peripheral T cell tolerance through the induction of AICD in T cells. The lack of AICD is often associated with autoimmune diseases as observed in IL-2−/−, IL-2Rα−/−, gld, and lpr mice (reviewed in ref. 32). The lower levels of IL-2 production in the IL-15Tg mouse prompted us to examine if IL-2-induced AICD still occurs in the IL-15Tg mouse. Interestingly, we observed that T cells from the IL-15Tg did not undergo apoptosis as efficiently as did the T cells from the wt mouse (Fig. 7B). We wondered if this phenotype was due to the alteration in the nature of CD4 T cells induced by the persistent presence of IL-15 in the IL-15Tg mouse or if it was due to an inhibition of IL-2 action by IL-15. To test these alternate hypotheses, a neutralizing antibody against human IL-15 was included in the assay. As shown in Fig. 7B, the antibody effectively restored the capacity of Tg CD4 T cells to undergo AICD, suggesting that this phenotype observed with IL-15Tg cells was reversible. This observation addressed two issues. Although CD4 cell numbers did not change in the IL-15Tg mouse, the overproduction of IL-15 has an effect on their function. Second, the restoration of AICD in the Tg CD4 T cells by the anti-IL-15 antibody indicated that the overproduction of IL-15 per se caused an alteration in the adaptive T cell immunity in these mice. Collectively, these pieces of evidence suggest that the IL-15 overproduction disrupted the normal balance between IL-2 and IL-15 and led to an abrogation of one of the critical responses, namely the AICD, required to maintain appropriate adaptive immunity. Thus, it is expected that a long-term observation of the IL-15Tg mice may demonstrate a disorder of certain immunological capacities in these mice.
Discussion
In this study, by generating a mouse overexpressing IL-15, we tried to clarify the in vivo functions of this cytokine that our group codiscovered in both innate and acquired immunity. We observed an expansion of NK, CD8CD44hi T, and γδ dendritic epidermal T cells, all of which are deficient in IL-15−/− or IL-15Rα−/− mice. We did not observe any change in the number of B cells or monocytes. Interestingly, a subset of NK-T cells expressing CD8αα′ emerged in this mouse, indicating that these are a novel cytokine-inducible cell. Ishida and coworkers (33) generated IL-2 Tg and IL-2Rα Tg mice, but neither displayed any apparent immunological phenotype with the exception that the IL-2Tg mice suffered from alopecia and pneumonia. However, when they crossed the IL-2 and IL-2Rα Tg mouse to generate an IL-2/IL-2Rα double Tg mouse (34), they observed the propagation of NK cells. Taken together with our IL-15Tg results, one may postulate that the combination of IL-2/IL-2Rα expression mimicked bona fide IL-15 function, as IL-15Rα is constitutively expressed on NK progenitor cells. Our view is that although the IL-2/IL-2R system plays a secondary role in NK cell activation, IL-15 is the primary cytokine in the development of NK cells. Consistent with this hypothesis is the fact that the IL-2−/− mouse had intact numbers of NK cells (16), whereas the IL-15−/− or IL-15Rα−/− mice lacked such cells and the IL-15Tg mouse manifested a marked increase in the number of NK cells in diverse organs.
Another interesting difference between the IL-2/IL-2Rα Tg and the IL-15Tg mouse is associated with CD8 T cells. Propagation of CD8 T cells observed with the IL-15Tg mouse was not observed with the IL-2 Tg mouse or the IL-2/IL-2Rα double Tg mouse. Incidentally, the IL-2Tg mouse expressed 10–500 pM IL-2 in the serum (34), a value comparable to that of IL-15 in our IL-15Tg mouse. Therefore, the differences between IL-2 and IL-15 in the propagation of CD8 T cells cannot be attributed to the differences in the concentrations of each lymphokine in the body fluids. This suggests that the signal transduced by the IL-15Rαβγ complex and that by the IL-2Rαβγ complex might be qualitatively different, despite the sharing of the βγ classical signaling receptor components between these two receptor systems.
The expansion of the CD8+ NK-T cells is another interesting feature associated with the IL-15Tg mouse. Although NK-T cells found under normal conditions express CD4 antigen, the existence of CD8-type NK-T cells raises a question concerning the current understanding of the ontogeny of the NK-T lineage cells. We confirmed that CD8 NK-T cells did not produce IL-4 upon antigen-like stimulation, although they produced γ-IFN, an observation suggesting a functional difference between CD4 and CD8 NK-T cells.
Propagation of CD8+ CD44hi T cells in IL-15Tg mice raises another question. In the past, Zhang et al. (19) demonstrated a similar phenomenon upon repeated injections of recombinant IL-15 into wt mice. Furthermore, a recent demonstration by Ku et al. (21) indicated that IL-15 supports the survival of functional memory CD8 cells. In our study, although the mice were kept in a pathogen-free environment, we observed the propagation of such cells and noted that these cells lack the expression of early T cell activation markers such as CD25 or CD69. It is possible that either an autoantigen or some environmental antigens may have primed these CD44hiCD8 T cells in the IL-15Tg mouse. So far, we have not observed any signs of autoimmune phenomenon in the IL-15Tg mouse on the C57BL6 background. Perhaps crossing of the IL-15Tg onto a different genetic background may be needed to further address this issue.
We observed in the IL-15Tg an augmented immunity against infections that are associated with NK and CTL activities. In particular, experiments using the vaccinia virus indicated that IL-15Tg mouse is more efficient in clearing the virus in the early phase of infection. As noted above, IL-15 plays a significant role in the proliferation of select lymphocytes including NK cells, CD8 NK-T cells, and especially memory phenotype CD8 T cells. This cytokine also acts to extend the survival of lymphocytes by inhibiting apoptotic death (31). In particular, ex vivo studies involving CD4 T cells from the IL-15Tg mouse demonstrated the lack of IL-2-induced AICD, a form of T cell suicide required to maintain peripheral self-tolerance. Of interest is the additional observation that the inclusion of an anti-IL-15 antibody resulted in the restoration of the ability of IL-2 to induce AICD with CD4 T cells from the IL-15Tg mouse. Thus, the IL-15Tg mouse system facilitated the elucidation of a new role for IL-15 in the regulation of peripheral T cell tolerance. IL-2 and IL-15 have conflicting effects not only in IL-2-induced AICD but also in some other CD4 T cells responses (S.D. and Y.T., unpublished observation). In a different system, Ku and coworkers (21) demonstrated such opposing effects of IL-2 and IL-15 in the maintenance of functional CD8 memory T cells. Again, IL-15 was involved in the survival and maintenance of lymphocytes, whereas IL-2 seemed to reduce the number of CD8 memory T cells. Thus, in adaptive immunity, IL-2 favors the elimination of lymphocytes by inducing cell death, and IL-15 contributes to the survival and perpetuation of T cells. However, it should be noted that this potential of IL-15 can be dangerous to the body, as the survival of autoreactive T cells can led to the onset of an autoimmune disease. For example, it was proposed that IL-15 might be associated with the cause of rheumatoid arthritis (35). Thus again, the proper balance between IL-2 and IL-15 is an important element in the maintenance of appropriate immunity. In summary, both IL-2 and especially IL-15 are critical for NK cell-mediated innate immunity. However, they have competing roles in adaptive immunity with IL-2 responsible for tolerance to self-antigens, whereas IL-15 plays a major role in the perpetuation of memory lymphocytes during normal immune responses.
Acknowledgments
We extend special regards to Dr. Mayumi Naramura, Laboratory of Immunology, National Institute of Infectious and Allergic Diseases, and Dr. Susan B. Silk, National Cancer Institute, for critical suggestions and advice concerning the generation and analysis of the transgenic mice. We thank Dr. Jeffrey Schlom, Laboratory of Tumor Biology and Immunology, National Cancer Institute, for the kind supply of the Syeth strain of the vaccinia virus.
Abbreviations
- Tg
transgenic
- wt
wild type
- SP
signal peptide
- AICD
activation-induced cell death
- FCM
flow cytometry
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200363097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200363097
References
- 1.Burton J D, Bamford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamford R N, Grant A J, Burton J D, Peters C, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, et al. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 4.Giri J G, Kumaki S, Ahdieh M, Friend D J, Loomis A, Shanebeck K, DuBose R, Cosman D, Park L S, Anderson D M. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldmann T A, Tagaya Y. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Bamford R N, Battiata A P, Burton J D, Sharma H, Waldmann T A. Proc Natl Acad Sci USA. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelbaum D, Mohamadzadeh M, Bergstresser P R, Sugamura K, Takashima A. J Invest Dermatol. 1995;105:837–843. doi: 10.1111/1523-1747.ep12326630. [DOI] [PubMed] [Google Scholar]
- 8.Blauvelt A, Asada H, Klaus-Kovtun V, Altman D J, Lucey D R, Katz S I. J Invest Dermatol. 1996;106:1047–1052. doi: 10.1111/1523-1747.ep12338641. [DOI] [PubMed] [Google Scholar]
- 9.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, Waldmann T A, Taniguchi T, Taki S. Nature (London) 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 10.Ohteki T, Ho S, Suzuki H, Mak T W, Ohashi P S. J Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- 11.Ohteki T, Yoshida H, Matsuyama T, Duncan G S, Mak T W, Ohashi P S. J Exp Med. 1998;187:967–972. doi: 10.1084/jem.187.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tagaya Y, Bamford R N, DeFilippis A P, Waldmann T A. Immunity. 1996;4:329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 13.Tagaya Y, Burton J D, Miyamoto Y, Waldmann T A. EMBO J. 1996;15:4928–4939. [PMC free article] [PubMed] [Google Scholar]
- 14.Willerford D M, Chen J, Ferry J A, Davidson L, Ma A, Alt F W. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 15.Lodolce J P, Boone D L, Chai S, Swain R E, Dassopoulos T, Trettin S, Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 16.Kundig T M, Schorle H, Bachmann M F, Hengartner H, Zinkernagel R M, Horak I. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy M K, Glaccum M, Brown S N, Butz E A, Viney J L, Embers M, Matsuki N, Charrier K, Sedger L, Willis C R, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma A, Boone D L, Lodolce J P. J Exp Med. 2000;191:753–756. doi: 10.1084/jem.191.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 20.Lenardo M J. Nature (London) 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 21.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 22.Uetsuki T, Naito A, Nagata S, Kaziro Y. J Biol Chem. 1989;264:5791–5798. [PubMed] [Google Scholar]
- 23.Matzinger P. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 24.Bamford R N, DeFilippis A P, Azimi N, Kurys G, Waldmann T A. J Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- 25.Tagaya Y, Kurys G, Thies T A, Losi J M, Azimi N, Hanover J A, Bamford R N, Waldmann T A. Proc Natl Acad Sci USA. 1997;94:14444–14449. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carson W E, Giri J G, Lindemann M J, Linett M L, Ahdieh M, Paxton R, Anderson D, Eisenman J, Grabstein K, Caligiuri M A. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puzanov I J, Bennett M, Kumar V. J Immunol. 1996;157:4282–4285. [PubMed] [Google Scholar]
- 28.Nishimura H, Hiromatsu K, Kobayashi N, Grabstein K H, Paxton R, Sugamura K, Bluestone J A, Yoshikai Y. J Immunol. 1996;156:663–669. [PubMed] [Google Scholar]
- 29.Bendelac A, Killeen N, Littman D R, Schwartz R H. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 30.Ballas Z K, Rasmussen W. J Immunol. 1993;150:17–30. [PubMed] [Google Scholar]
- 31.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H, Kunzendorf U. Nat Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 32.Lenardo M, Chan K M, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 33.Ishida Y, Nishi M, Taguchi O, Inaba K, Minato N, Kawaichi M, Honjo T. Int Immunol. 1989;1:113–120. doi: 10.1093/intimm/1.2.113. [DOI] [PubMed] [Google Scholar]
- 34.Ishida Y, Nishi M, Taguchi O, Inaba K, Hattori M, Minato N, Kawaichi M, Honjo T. J Exp Med. 1989;170:1103–1115. doi: 10.1084/jem.170.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McInnes I B, Liew F Y. Immunol Today. 1998;19:75–79. doi: 10.1016/s0167-5699(97)01205-x. [DOI] [PubMed] [Google Scholar]