Abstract
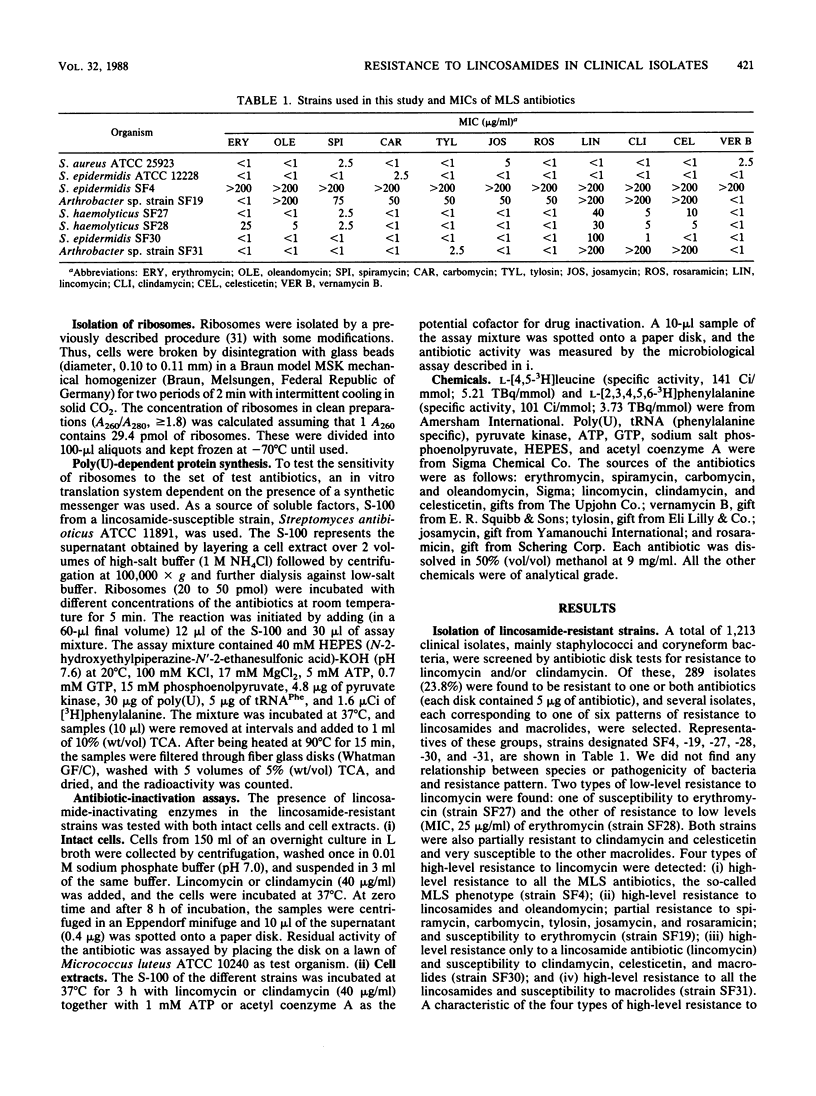
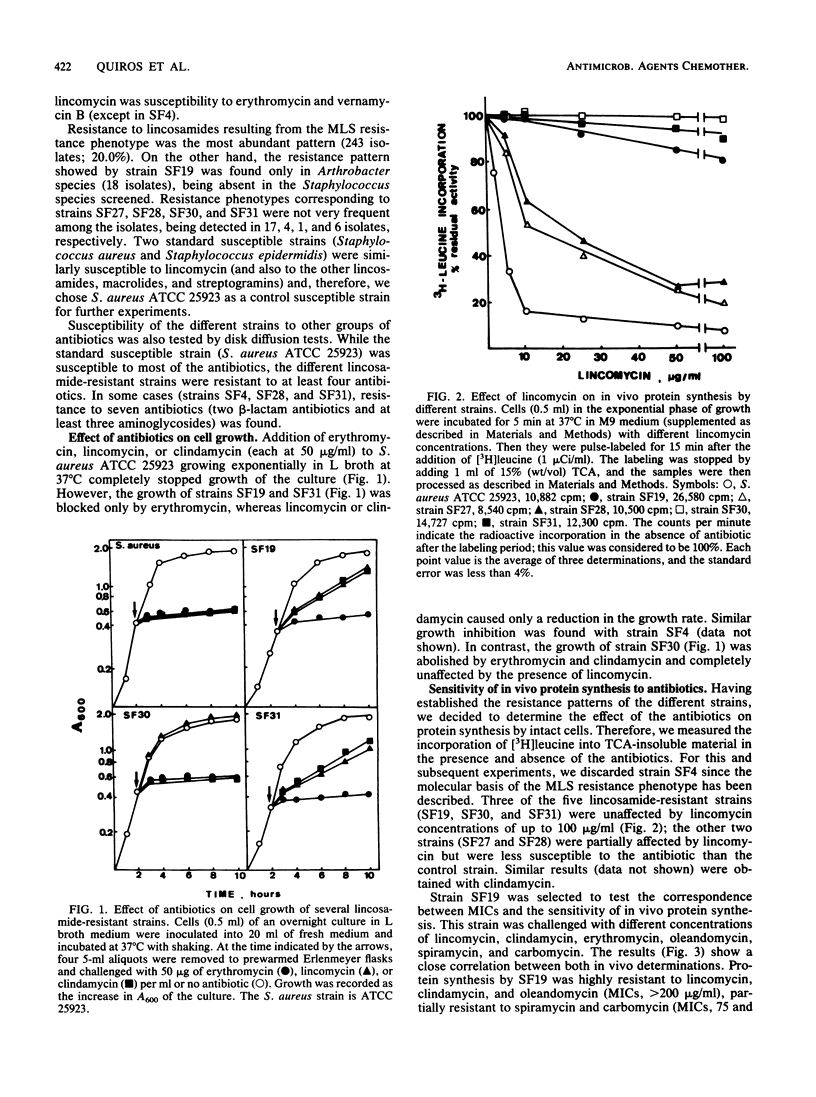
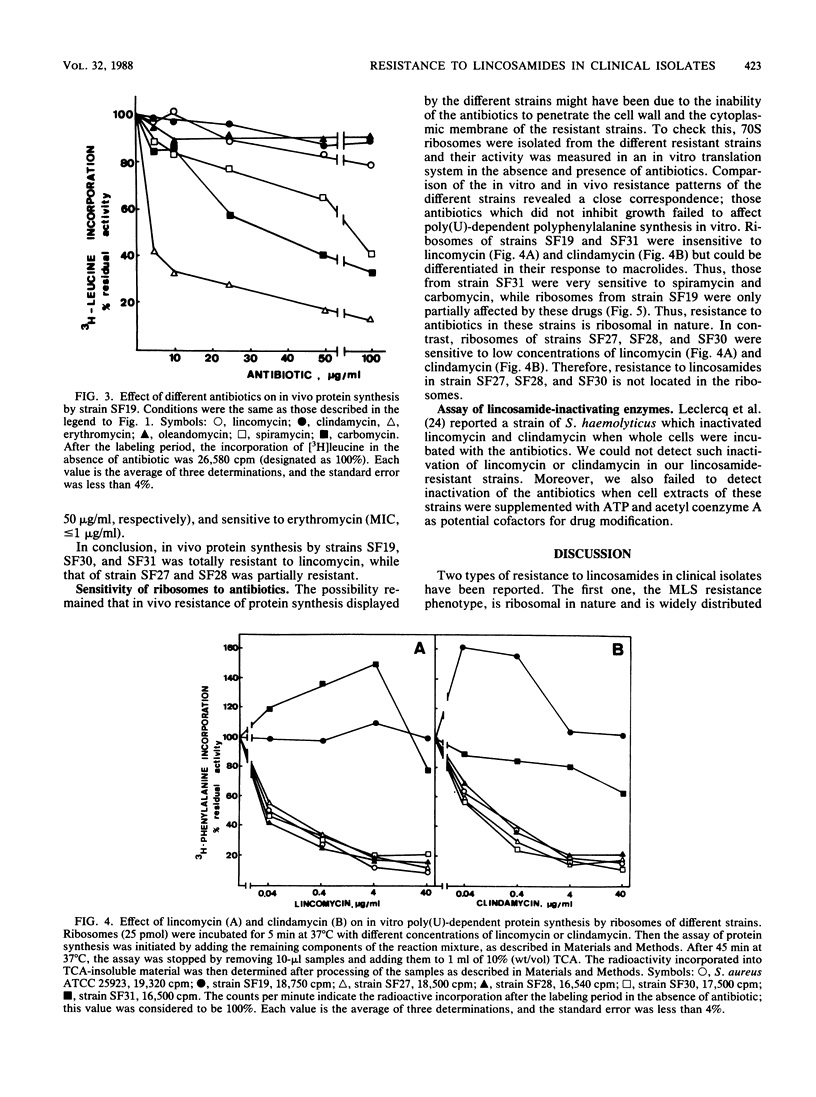
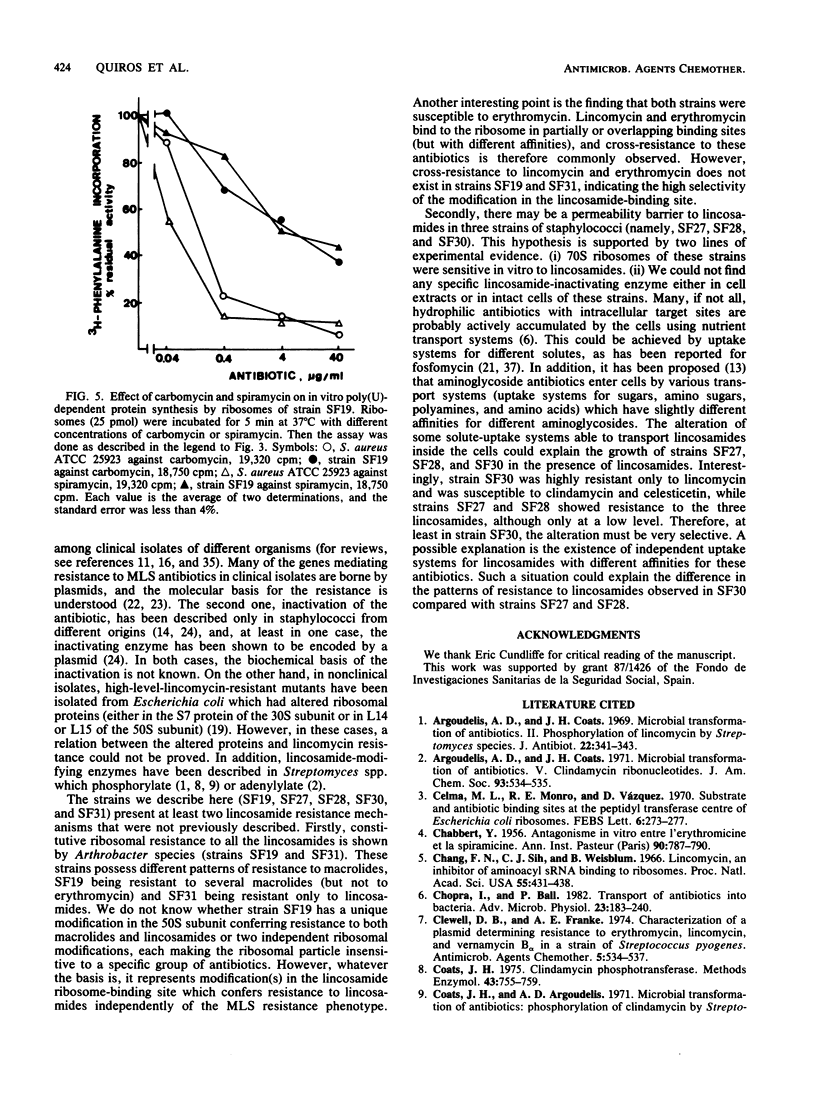
Clinical isolates of Staphylococcus and Arthrobacter spp. were screened for lincosamide resistance. Six different patterns of resistance were found. Strains designated SF27 and SF28 showed low-level resistance to lincosamides: one was susceptible to erythromycin (SF27) and the other was resistant (SF28). Analysis of ribosomes from the resistant strains in an in vitro poly(U)-dependent protein-synthesizing system showed that ribosomes of both strains were sensitive to lincomycin and clindamycin. Four patterns of high-level resistance to lincosamides were observed (strains SF4, SF19, SF30, and SF31). All of these except SF30 had ribosomes which were highly resistant in vitro to the antibiotics and showed a close correlation with results of the in vivo experiments. In vivo protein synthesis by strain SF30 was resistant to lincomycin and sensitive to clindamycin, whereas the ribosomes were sensitive when assayed in vitro. Lincosamide-inactivating enzymes were not detected in cell extracts of the six resistant strains. Strains SF19 and SF31 demonstrated two ribosome-mediated lincosamides resistance mechanisms that were not previously reported. Both strains were highly resistant to lincosamides and susceptible to erythromycin, but SF19 was also highly resistant to oleandomycin and partially resistant to various macrolides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argoudelis A. D., Coats J. H. Microbial transformation of antibiotics. II. Phosphorylation of lincomycin by Streptomyces species. J Antibiot (Tokyo) 1969 Jul;22(7):341–343. doi: 10.7164/antibiotics.22.341. [DOI] [PubMed] [Google Scholar]
- Argoudelis A. D., Coats J. H. Microbial transformation of antibiotics. V. Clindamycin ribonucleotides. J Am Chem Soc. 1971 Jan 27;93(2):534–535. doi: 10.1021/ja00731a047. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y. Antagonisme in vitro entre l'érythromycine et la spiramycine. Ann Inst Pasteur (Paris) 1956 Jun;90(6):787–790. [PubMed] [Google Scholar]
- Celma M. L., Monro R. E., Vazquez D. Substrate and antibiotic binding sites at the peptidyl transferase centre of E. coli ribosomes. FEBS Lett. 1970 Feb 16;6(3):273–277. doi: 10.1016/0014-5793(70)80076-x. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Sih C. J., Weisblum B. Lincomycin, an inhibitor of aminoacyl sRNA binding to ribosomes. Proc Natl Acad Sci U S A. 1966 Feb;55(2):431–438. doi: 10.1073/pnas.55.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Ball P. Transport of antibiotics into bacteria. Adv Microb Physiol. 1982;23:183–240. doi: 10.1016/s0065-2911(08)60338-0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats J. H., Argoudelis A. D. Microbial transformation of antibiotics: phosphorylation of clindamycin by Streptomyces coelicolor Müller. J Bacteriol. 1971 Oct;108(1):459–464. doi: 10.1128/jb.108.1.459-464.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats J. H. Clindamycin hosphotransferase. Methods Enzymol. 1975;43:755–759. doi: 10.1016/0076-6879(75)43142-1. [DOI] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Courvalin P., Ounissi H., Arthur M. Multiplicity of macrolide-lincosamide-streptogramin antibiotic resistance determinants. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):91–100. doi: 10.1093/jac/16.suppl_a.91. [DOI] [PubMed] [Google Scholar]
- Coyle M. B., Minshew B. H., Bland J. A., Hsu P. C. Erythromycin and clindamycin resistance in Corynebacterium diphtheriae from skin lesions. Antimicrob Agents Chemother. 1979 Oct;16(4):525–527. doi: 10.1128/aac.16.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damper P. D., Epstein W. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob Agents Chemother. 1981 Dec;20(6):803–808. doi: 10.1128/aac.20.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devriese L. A. Two new types of resistance to lincomycin in pathogenic staphylococci from animals. Ann Microbiol (Paris) 1980 Nov-Dec;131B(3):261–266. [PubMed] [Google Scholar]
- Docherty A., Grandi G., Grandi R., Gryczan T. J., Shivakumar A. G., Dubnau D. Naturally occurring macrolide-lincosamide-streptogramin B resistance in Bacillus licheniformis. J Bacteriol. 1981 Jan;145(1):129–137. doi: 10.1128/jb.145.1.129-137.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. Translational attenuation: the regulation of bacterial resistance to the macrolide-lincosamide-streptogramin B antibiotics. CRC Crit Rev Biochem. 1984;16(2):103–132. doi: 10.3109/10409238409102300. [DOI] [PubMed] [Google Scholar]
- Dutta G. N., Devriese L. A. Macrolide-lincosamide-streptogramin resistance patterns in Clostridium perfringens from animals. Antimicrob Agents Chemother. 1981 Feb;19(2):274–278. doi: 10.1128/aac.19.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. Y., Weisblum B. 23S ribosomal ribonucleic acid of macrolide-producing streptomycetes contains methylated adenine. J Bacteriol. 1979 Mar;137(3):1464–1467. doi: 10.1128/jb.137.3.1464-1467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel H., Piepersberg W., Böck A. Analysis of lincomycin resistance mutations in Escherichia coli. Mol Gen Genet. 1979 Feb 1;169(3):345–347. doi: 10.1007/BF00382280. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Ishitsuka H., Kaji A. Comparative studies on the mechanism of action of lincomycin, streptomycin, and erythromycin. Biochem Biophys Res Commun. 1969 Oct 22;37(3):499–504. doi: 10.1016/0006-291x(69)90943-7. [DOI] [PubMed] [Google Scholar]
- Kahan F. M., Kahan J. S., Cassidy P. J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974 May 10;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B., Fahnestock S. R., Nomura M. Alteration of 23 S ribosomal RNA and erythromycin-induced resistance to lincomycin and spiramycin in Staphylococcus aureus. J Mol Biol. 1973 Feb 15;74(1):67–72. doi: 10.1016/0022-2836(73)90355-0. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Carlier C., Duval J., Courvalin P. Plasmid-mediated resistance to lincomycin by inactivation in Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1985 Sep;28(3):421–424. doi: 10.1128/aac.28.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monro R. E., Vazquez D. Ribosome-catalysed peptidyl transfer: effects of some inhibitors of protein synthesis. J Mol Biol. 1967 Aug 28;28(1):161–165. doi: 10.1016/s0022-2836(67)80085-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E. MLS-resistance determinants in Staphylococcus aureus and their molecular evolution. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):101–110. doi: 10.1093/jac/16.suppl_a.101. [DOI] [PubMed] [Google Scholar]
- Parisi J. T., Robbins J., Lampson B. C., Hecht D. W. Characterization of a macrolide, lincosamide, and streptogramin resistance plasmid in Staphylococcus epidermidis. J Bacteriol. 1981 Nov;148(2):559–564. doi: 10.1128/jb.148.2.559-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Dublanchet A., Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979 Jan;139(1):97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- Roberts A. N., Hudson G. S., Brenner S. An erythromycin-resistance gene from an erythromycin-producing strain of Arthrobacter sp. Gene. 1985;35(3):259–270. doi: 10.1016/0378-1119(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Skeggs P. A., Thompson J., Cundliffe E. Methylation of 16S ribosomal RNA and resistance to aminoglycoside antibiotics in clones of Streptomyces lividans carrying DNA from Streptomyces tenjimariensis. Mol Gen Genet. 1985;200(3):415–421. doi: 10.1007/BF00425725. [DOI] [PubMed] [Google Scholar]
- Skinner R., Cundliffe E., Schmidt F. J. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J Biol Chem. 1983 Oct 25;258(20):12702–12706. [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Distribution of an inducible hexose-phosphate transport system among various bacteria. J Bacteriol. 1973 Nov;116(2):1079–1081. doi: 10.1128/jb.116.2.1079-1081.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



