Abstract
AIMS—This study was undertaken to confirm the distribution and expression of the molecule CD44 on human corneas under normal and pathological conditions. METHODS—Fifty eight corneal buttons from adult patients suffering from various corneal diseases and four normal corneas were included in this study. Frozen sections were stained immunohistochemically with monoclonal antibodies against human CD44 using an APAAP method and observed under a light microscope. RESULTS—In normal corneas CD44 was predominantly expressed on the membranes of basal epithelial cells and on the keratocytes, as well as on the vascular endothelial cells of the corneal limbi, but was not expressed on corneal endothelial cells. Enhanced expression of CD44 was observed on the epithelium of corneas with inflammation and allograft rejection. In a number of abnormal conditions including allograft rejection, corneal trauma, primary and secondary corneal endothelial decompensation the remaining endothelial cells stained positively for CD44. However, in some corneas of keratitis, keratoconus, and dystrophy the endothelium which appeared relatively integral in morphology and amount remained CD44 negative. CONCLUSIONS—These results suggest that CD44, the hyaluronate receptor, may play an important role in corneal cell-cell and cell-matrix interactions. Its regulation is closely related to corneal inflammatory reactions. The induction of CD44 on corneal endothelium might play a potential role in compensatory processes when corneal endothelial cells are injured.
Full Text
The Full Text of this article is available as a PDF (191.8 KB).
Figure 1 .
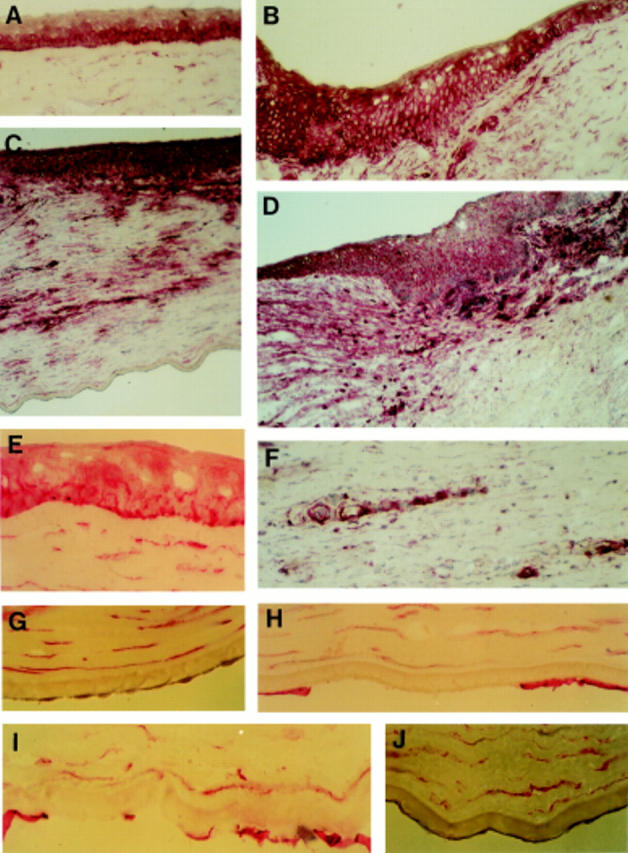
Alkaline phosphatase anti-alkaline phosphatase immunostaining for CD44 in human corneal sections. Original magnification × 200 for (A), (B), (C), (D) and (F); and × 400 for the others. (A) Epithelial cell staining in normal central region; (B) epithelial cell staining in normal limbus; (C) section from a cornea with keratitis showing intense staining in all epithelial layers, but no staining on endothelial cells; (D) section from a cornea with allograft rejection showing the staining of epithelium in the region where graft and receptor corneas adjoin; (E) epithelial cell staining in Fuchs' endothelial dystrophy; (F) CD44 staining on neovascular endothelium and infiltrating cells in cornea with keratitis; (G) normal corneal endothelial cells showing no staining for CD44; (H) section from a cornea with pseudophakic bullous keratopathy showing positive staining on the remaining endothelial cells; (I) section from a cornea with Fuchs' endothelial dystrophy showing the CD44 staining on the remaining endothelial cells; and (J) section from a cornea with keratoconus showing negative staining of endothelial cells for CD44.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbasi A. M., Chester K. A., Talbot I. C., Macpherson A. S., Boxer G., Forbes A., Malcolm A. D., Begent R. H. CD44 is associated with proliferation in normal and neoplastic human colorectal epithelial cells. Eur J Cancer. 1993;29A(14):1995–2002. doi: 10.1016/0959-8049(93)90461-n. [DOI] [PubMed] [Google Scholar]
- Alho A. M., Underhill C. B. The hyaluronate receptor is preferentially expressed on proliferating epithelial cells. J Cell Biol. 1989 Apr;108(4):1557–1565. doi: 10.1083/jcb.108.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Asari A., Miyauchi S., Takahashi T., Kohno K., Uchiyama Y. Localization of hyaluronic acid, chondroitin sulfate, and CD44 in rabbit cornea. Arch Histol Cytol. 1992 Dec;55(5):503–511. doi: 10.1679/aohc.55.503. [DOI] [PubMed] [Google Scholar]
- Camp R. L., Kraus T. A., Puré E. Variations in the cytoskeletal interaction and posttranslational modification of the CD44 homing receptor in macrophages. J Cell Biol. 1991 Dec;115(5):1283–1292. doi: 10.1083/jcb.115.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A. Characterization of the class III collagen receptor, a phosphorylated, transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem. 1988 Mar 25;263(9):4193–4201. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Elner V. M., Dutt S., Pavilack M. A., Sugar A., Foster C. S., Elner S. G. Intercellular adhesion molecule-1 (ICAM-1) and HLA-DR antigens in herpes keratitis. Ophthalmology. 1992 Sep;99(9):1400–1407. doi: 10.1016/s0161-6420(92)31801-9. [DOI] [PubMed] [Google Scholar]
- Foets B. J., van den Oord J. J., Volpes R., Missotten L. In situ immunohistochemical analysis of cell adhesion molecules on human corneal endothelial cells. Br J Ophthalmol. 1992 Apr;76(4):205–209. doi: 10.1136/bjo.76.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S. B., Fawcett J., Jackson D. G., Collins I., Gatter K. C., Harris A. L., Gearing A., Simmons D. L. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 1994 Aug 15;54(16):4539–4546. [PubMed] [Google Scholar]
- Galandrini R., De Maria R., Piccoli M., Frati L., Santoni A. CD44 triggering enhances human NK cell cytotoxic functions. J Immunol. 1994 Nov 15;153(10):4399–4407. [PubMed] [Google Scholar]
- Goldberg M. F., Ferguson T. A., Pepose J. S. Detection of cellular adhesion molecules in inflamed human corneas. Ophthalmology. 1994 Jan;101(1):161–168. doi: 10.1016/s0161-6420(94)31370-4. [DOI] [PubMed] [Google Scholar]
- Günthert U. CD44: a multitude of isoforms with diverse functions. Curr Top Microbiol Immunol. 1993;184:47–63. doi: 10.1007/978-3-642-78253-4_4. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hale L. P., Patton K. L., Martin M. E., McCallum R. M. Measurement of an adhesion molecule as an indicator of inflammatory disease activity. Up-regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritis. Arthritis Rheum. 1991 Nov;34(11):1434–1443. doi: 10.1002/art.1780341115. [DOI] [PubMed] [Google Scholar]
- Ito M., Watanabe M., Ihara T., Kamiya H., Sakurai M. Increased expression of adhesion molecules (CD54, CD29 and CD44) on fibroblasts infected with cytomegalovirus. Microbiol Immunol. 1995;39(2):129–133. doi: 10.1111/j.1348-0421.1995.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992 Feb;116(3):817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. A., Haines G. K., Harlow L. A., Koch A. E. Adhesion molecule expression in human synovial tissue. Arthritis Rheum. 1993 Feb;36(2):137–146. doi: 10.1002/art.1780360203. [DOI] [PubMed] [Google Scholar]
- Koopman G., Heider K. H., Horst E., Adolf G. R., van den Berg F., Ponta H., Herrlich P., Pals S. T. Activated human lymphocytes and aggressive non-Hodgkin's lymphomas express a homologue of the rat metastasis-associated variant of CD44. J Exp Med. 1993 Apr 1;177(4):897–904. doi: 10.1084/jem.177.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R., Kireta S., Carter J. K., Grooby W. L., Rao M. M., Russ G. R. Expression of alternatively spliced CD44 mRNA in sheep renal allografts. Transplant Proc. 1995 Jun;27(3):2170–2171. [PubMed] [Google Scholar]
- Kuppner M. C., Liversidge J., McKillop-Smith S., Lumsden L., Forrester J. V. Adhesion molecule expression in acute and fibrotic sympathetic ophthalmia. Curr Eye Res. 1993 Oct;12(10):923–934. doi: 10.3109/02713689309020399. [DOI] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Kincade P. W. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Terpe H. J., Stauder R., Marston W. L., Stark H., Günthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994 Jan;124(1-2):71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Loetscher H., Stueber D., Gehr G., Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med. 1993 May 1;177(5):1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley J. L., Dailey M. O. Regulation of adhesion molecule expression by CD8 T cells in vivo. I. Differential regulation of gp90MEL-14 (LECAM-1), Pgp-1, LFA-1, and VLA-4 alpha during the differentiation of cytotoxic T lymphocytes induced by allografts. J Immunol. 1992 Apr 15;148(8):2348–2356. [PubMed] [Google Scholar]
- Mobley J. L., Rigby S. M., Dailey M. O. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J Immunol. 1994 Dec 15;153(12):5443–5452. [PubMed] [Google Scholar]
- Mäenpä A., Jäskeläinen J., Carpén O., Patarroyo M., Timonen T. Expression of integrins and other adhesion molecules on NK cells; impact of IL-2 on short- and long-term cultures. Int J Cancer. 1993 Mar 12;53(5):850–855. doi: 10.1002/ijc.2910530524. [DOI] [PubMed] [Google Scholar]
- Osada A., Nakashima H., Furue M., Tamaki K. Up-regulation of CD44 expression by tumor necrosis factor-alpha is neutralized by interleukin-10 in Langerhans cells. J Invest Dermatol. 1995 Jul;105(1):124–127. doi: 10.1111/1523-1747.ep12313437. [DOI] [PubMed] [Google Scholar]
- Penneys N. S. CD44 expression in normal and inflamed skin. J Cutan Pathol. 1993 Jun;20(3):250–253. doi: 10.1111/j.1600-0560.1993.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Philipp W. Leukocyte adhesion molecules in rejected corneal allografts. Graefes Arch Clin Exp Ophthalmol. 1994 Feb;232(2):87–95. doi: 10.1007/BF00171669. [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Nussenzweig R. S., Romero P., Zavala F. The in vivo cytotoxic activity of CD8+ T cell clones correlates with their levels of expression of adhesion molecules. J Exp Med. 1992 Apr 1;175(4):895–905. doi: 10.1084/jem.175.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg W. M., Prince C., Kaklamanis L., Fox S. B., Jackson D. G., Simmons D. L., Chapman R. W., Trowell J. M., Jewell D. P., Bell J. I. Increased expression of CD44v6 and CD44v3 in ulcerative colitis but not colonic Crohn's disease. Lancet. 1995 May 13;345(8959):1205–1209. doi: 10.1016/s0140-6736(95)91991-0. [DOI] [PubMed] [Google Scholar]
- Salmi M., Grön-Virta K., Sointu P., Grenman R., Kalimo H., Jalkanen S. Regulated expression of exon v6 containing isoforms of CD44 in man: downregulation during malignant transformation of tumors of squamocellular origin. J Cell Biol. 1993 Jul;122(2):431–442. doi: 10.1083/jcb.122.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sconocchia G., Titus J. A., Segal D. M. CD44 is a cytotoxic triggering molecule in human peripheral blood NK cells. J Immunol. 1994 Dec 15;153(12):5473–5481. [PubMed] [Google Scholar]
- Uff C. R., Reid S. D., Wood R. F., Pockley A. G. CD44 expression on enterocytes: an indicator of rejection following rat small bowel transplantation. Transplant Proc. 1994 Jun;26(3):1553–1553. [PubMed] [Google Scholar]
- Underwood P. A., Bennett F. A. The effect of extracellular matrix molecules on the in vitro behavior of bovine endothelial cells. Exp Cell Res. 1993 Apr;205(2):311–319. doi: 10.1006/excr.1993.1091. [DOI] [PubMed] [Google Scholar]
- Vorkauf W., Vorkauf M., Nölle B., Duncker G. Adhesion molecules in normal and pathological corneas. An immunohistochemical study using monoclonal antibodies. Graefes Arch Clin Exp Ophthalmol. 1995 Apr;233(4):209–219. doi: 10.1007/BF00183594. [DOI] [PubMed] [Google Scholar]
- Willerford D. M., Hoffman P. A., Gallatin W. M. Expression of lymphocyte adhesion receptors for high endothelium in primates. Anatomic partitioning and linkage to activation. J Immunol. 1989 May 15;142(10):3416–3422. [PubMed] [Google Scholar]
- Woodruff J. J., Clarke L. M., Chin Y. H. Specific cell-adhesion mechanisms determining migration pathways of recirculating lymphocytes. Annu Rev Immunol. 1987;5:201–222. doi: 10.1146/annurev.iy.05.040187.001221. [DOI] [PubMed] [Google Scholar]


