Abstract
AIMS—Administration of unfractionated retinal antigen(s) (retinal extract, RE) suppresses RE induced experimental autoimmune uveoretinitis (EAU) and offers a potential therapeutic alternative to non-specific immunosuppressive therapies for posterior uveitis and autoimmune diseases. S-Ag and interphotoreceptor retinoid binding protein (IRBP) are two major autoantigens within soluble RE. It was aimed to assess, firstly, as has previously been shown with S-Ag, if IRBP can induce intranasal tolerance and, secondly, the contribution of both these major autoantigens to tolerance induction by whole RE. METHODS—Animals were tolerised by intranasal administration with S-Ag or IRBP, either alone or in combination, or RE before immunisation with either IRBP or RE. Control animals were administered nasally either PBS or MBP. Daily clinical responses were recorded biomicroscopically and histological grades were obtained using a semiquantitative scoring system. Weekly serum antibody levels to retinal antigens were measured by ELISA and delayed hypersensitivity responses (DTH) were assessed by skin reactivity to intradermal inoculation with retinal or non-specific antigens. RESULTS—Microgram doses of IRBP successfully suppressed both clinically and histologically IRBP induced EAU. This suppression was accompanied by reduced antigen specific DTH reactivity but maintained T cell dependent (IgG2a) antibody responses. Furthermore, combined S-Ag and IRBP administration afforded equal suppression of RE induced EAU when compared with RE therapy alone. Suppression of RE induced EAU was not achieved with administration of a non-retinal specific autoantigen, MBP. Although individually, both S-Ag and IRBP suppressed RE induced EAU, whole RE was unable to protect against IRBP induced disease. CONCLUSIONS—Intranasal administration of IRBP suppressed IRBP induced EAU in the Lewis rat. S-Ag and IRBP are the major contributors to the tolerogenicity within RE, despite the known uveogenicity of other retinal antigens within RE and induction of tolerance was retinal antigen specific. Furthermore, suppression induced by single antigen administration is antigen specific although concomitant bystander suppression may also play a role. RE was unable to protect against IRBP induced disease despite tolerogenic levels of antigen within RE. Although this may be due in part to a dose effect of either tolerising or immunising antigen, further investigation into the possible antigen dominance of IRBP or mucosal processing of combinations of antigens is necessary so that the full efficacy of mucosal tolerance therapy can be assessed.
Full Text
The Full Text of this article is available as a PDF (182.5 KB).
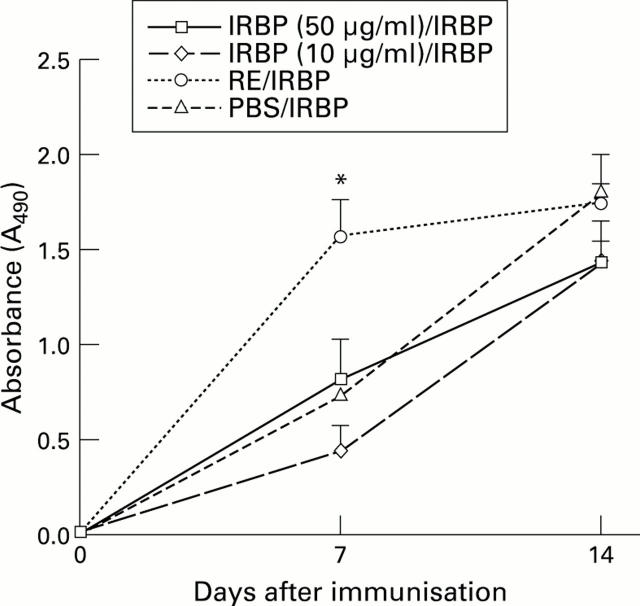
Figure 1 .
Antibody production in IRBP induced EAU in both controls and intranasal tolerised animals. There was no significant difference in total antibody levels (measured spectrophotometrically at OD490) except the group tolerised with retinal extract (RE) which had elevated total antibody titres (*p<0.05) on day 7. The increased antibody titre was predominantly IgG2a isotype. In the other groups antibody isotypes were also measured (IgM, IgG2a, and IgG1) and there was again no difference between the two groups with IgG2a generating the predominant antibody response (data not shown).
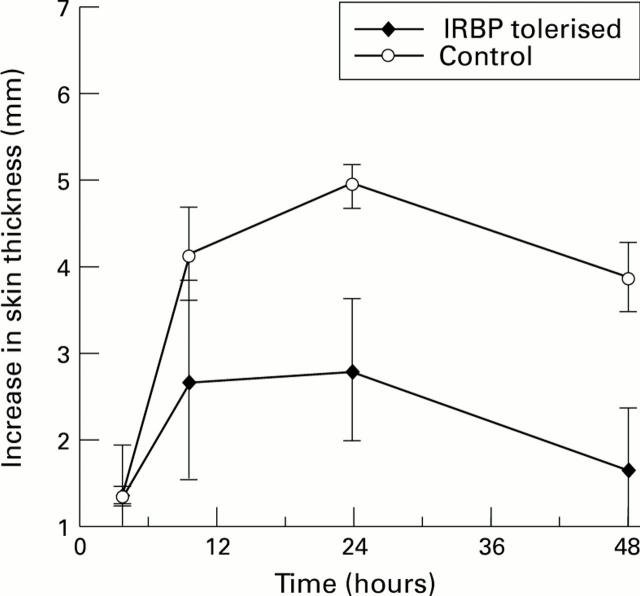
Figure 2 .
Delayed hypersensitivity reactivity (DTH) to retinal extract in both controls and IRBP tolerised animals in IRBP induced EAU. There was a significant reduction in DTH reactivity to retinal extract (p<0.05 at 24 hours) in animals tolerised with IRBP. This effect appears to be retinal antigen specific as reactivity to a non-specific protein, PPD, present in complete Freund's adjuvant within the immunising cocktail was equal in both groups (data not shown).
Figure 3 .
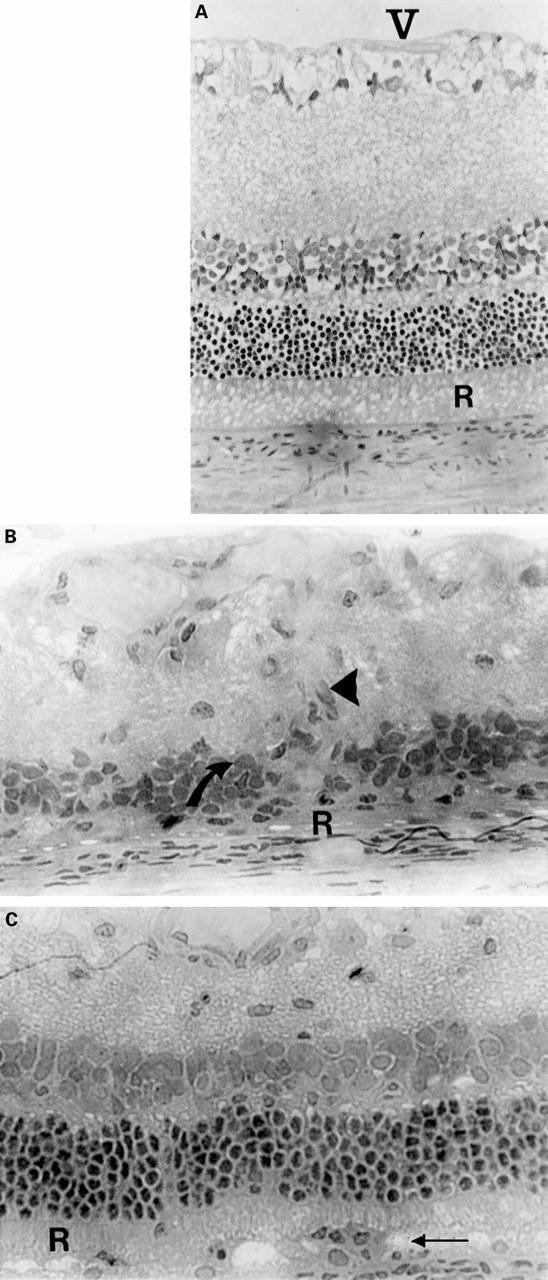
Administration of both S-Ag and IRBP intranasally protects against ROS damage in re-induced EAU. (A) Normal retinal morphology. V = vitreous, R = outer segments of rod photoreceptor cells (ROS). IRBP induced EAU results in a total loss of ROS (B, R) and destruction of both nuclear and ganglion cell layers of the retina (B, arrows). These animals also demonstrated fibrovascular membrane formation (arrowhead) as a result of intraocular inflammation. Administration of both tolerising doses of IRBP and S-Ag intranasally before immunisation with RE protects the ROS (C, R) although mononuclear leucocytic infiltration still occurs (C, arrow). The histological features are similar to the protection achieved with RE intranasal administration.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y., Inobe J., Marks R., Gonnella P., Kuchroo V. K., Weiner H. L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995 Jul 13;376(6536):177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994 Aug 26;265(5176):1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., Liversidge J., Forrester J. V. Immunomodulation of experimental autoimmune uveoretinitis: a model of tolerance induction with retinal antigens. Eye (Lond) 1994;8(Pt 1):52–59. doi: 10.1038/eye.1994.10. [DOI] [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., Liversidge J., Forrester J. V. Intranasal administration of retinal antigens suppresses retinal antigen-induced experimental autoimmune uveoretinitis. Immunology. 1994 Aug;82(4):625–631. [PMC free article] [PubMed] [Google Scholar]
- Dick A. D., Cheng Y. F., McKinnon A., Liversidge J., Forrester J. V. Nasal administration of retinal antigens suppresses the inflammatory response in experimental allergic uveoretinitis. A preliminary report of intranasal induction of tolerance with retinal antigens. Br J Ophthalmol. 1993 Mar;77(3):171–175. doi: 10.1136/bjo.77.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A. D. Experimental approaches to specific immunotherapies in autoimmune disease: future treatment of endogenous posterior uveitis? Br J Ophthalmol. 1995 Jan;79(1):81–88. doi: 10.1136/bjo.79.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P., Steel M., Liew F. Y., Mowat A. M. CD4+ but not CD8+ T cells are required for the induction of oral tolerance. Int Immunol. 1995 Mar;7(3):501–504. doi: 10.1093/intimm/7.3.501. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Obritsch W. F., Donoso L. A. Oral tolerance in experimental autoimmune uveoretinitis. Distinct mechanisms of resistance are induced by low dose vs high dose feeding protocols. J Immunol. 1993 Nov 15;151(10):5751–5761. [PubMed] [Google Scholar]
- Kamradt T., Soloway P. D., Perkins D. L., Gefter M. L. Pertussis toxin prevents the induction of peripheral T cell anergy and enhances the T cell response to an encephalitogenic peptide of myelin basic protein. J Immunol. 1991 Nov 15;147(10):3296–3302. [PubMed] [Google Scholar]
- Khoury S. J., Hancock W. W., Weiner H. L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992 Nov 1;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin C., Oliver J., Girn B., Holt B. J., Kees U. R., Thomas W. R., Holt P. G. Regulation of T-cell sensitization at epithelial surfaces in the respiratory tract: suppression of IgE responses to inhaled antigens by CD3+ Tcr alpha-/beta- lymphocytes (putative gamma/delta T cells). Immunology. 1991 Oct;74(2):234–239. [PMC free article] [PubMed] [Google Scholar]
- McMenamin C., Pimm C., McKersey M., Holt P. G. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994 Sep 23;265(5180):1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- McRae B. L., Vanderlugt C. L., Dal Canto M. C., Miller S. D. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995 Jul 1;182(1):75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D., Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993 Apr;23(4):935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- Melamed D., Friedman A. In vivo tolerization of Th1 lymphocytes following a single feeding with ovalbumin: anergy in the absence of suppression. Eur J Immunol. 1994 Sep;24(9):1974–1981. doi: 10.1002/eji.1830240906. [DOI] [PubMed] [Google Scholar]
- Metzler B., Wraith D. C. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int Immunol. 1993 Sep;5(9):1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- Nussenblatt R. B., Caspi R. R., Mahdi R., Chan C. C., Roberge F., Lider O., Weiner H. L. Inhibition of S-antigen induced experimental autoimmune uveoretinitis by oral induction of tolerance with S-antigen. J Immunol. 1990 Mar 1;144(5):1689–1695. [PubMed] [Google Scholar]
- Nussenblatt R. B., Rodrigues M. M., Wacker W. B., Cevario S. J., Salinas-Carmona M. C., Gery I. Cyclosporin a. Inhibition of experimental autoimmune uveitis in Lewis rats. J Clin Invest. 1981 Apr;67(4):1228–1231. doi: 10.1172/JCI110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo L. V., Miller-Rivero N. E., Chan C. C., Wiggert B., Nussenblatt R. B., Caspi R. R. Interleukin-2 treatment potentiates induction of oral tolerance in a murine model of autoimmunity. J Clin Invest. 1994 Oct;94(4):1668–1672. doi: 10.1172/JCI117511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. S., Harper N., Bevan D. J., Staines N. A. Suppression of collagen induced arthritis by oral administration of type II collagen: changes in immune and arthritic responses mediated by active peripheral suppression. Autoimmunity. 1993;16(3):189–199. doi: 10.3109/08916939308993327. [DOI] [PubMed] [Google Scholar]
- Vrabec T. R., Gregerson D. S., Dua H. S., Donoso L. A. Inhibition of experimental autoimmune uveoretinitis by oral administration of S-antigen and synthetic peptides. Autoimmunity. 1992;12(3):175–184. doi: 10.3109/08916939209148457. [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., He B., Qiao J., Link H. Suppression of experimental autoimmune myasthenia gravis and experimental allergic encephalomyelitis by oral administration of acetylcholine receptor and myelin basic protein: double tolerance. J Neuroimmunol. 1995 Dec;63(1):79–86. doi: 10.1016/0165-5728(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Mackin G. A., Matsui M., Orav E. J., Khoury S. J., Dawson D. M., Hafler D. A. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993 Feb 26;259(5099):1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- Wildner G., Thurau S. R. Orally induced bystander suppression in experimental autoimmune uveoretinitis occurs only in the periphery and not in the eye. Eur J Immunol. 1995 May;25(5):1292–1297. doi: 10.1002/eji.1830250524. [DOI] [PubMed] [Google Scholar]