Abstract
AIMS/BACKGROUND—Epiretinal membranes (ERMs) arise from a variety of causes or, in some cases, for unknown reasons. Once established, ERMs tend to progress, becoming more extensive and exerting increasing traction along the inner surface of the retina. One possible cause for their progression is the production of growth factors by cells within ERMs that may provide autocrine or paracrine stimulation. Platelet derived growth factor (PDGF) and its receptors have been localised to cells of ERMs and may play such a role. In this study, comparative data were sought for several other growth factors that have been implicated in ERM formation. METHODS—Immunohistochemical staining of ERMs was done for PDGF-A, PDGF-B, basic fibroblast growth factor (bFGF), three isoforms of transforming growth factor β (TGF-β), and vascular endothelial growth factor (VEGF) and its receptors, flt-1 and flk-1/KDR. Expression of flt-1 and flk-1/KDR was examined in cultured retinal pigmented epithelial (RPE) cells and retinal glia from postmortem eyes by immunohistochemistry and by reverse transcription coupled to polymerase chain reaction (RT-PCR). RESULTS—Staining was most intense and most frequently observed for VEGF and PDGF-A, both in vascular and avascular ERMs. The majority of cells stained for VEGF in nine of 11 (81.8%) diabetic ERMs and in 14 of 24 (58.3%) proliferative vitreoretinopathy ERMs. The receptors for VEGF, flt-1, and flk-1/KDR were also identified on cells in ERMs and on cultured RPE cells. By RT-PCR, mRNA for flt-1 was identified in RPE cells and retinal glia, and mRNA for flk-1/KDR was identified in RPE cells. CONCLUSIONS—These data show that VEGF and its receptors are localised to both vascular and avascular ERMs and suggest that VEGF, like PDGF-A, may be an autocrine and paracrine stimulator that may contribute to progression of vascular and avascular ERMs.
Full Text
The Full Text of this article is available as a PDF (247.5 KB).
Figure 1 .
VEGF immunostaining in PDR, PVR, and idiopathic ERMs is visualised with a red reaction product. (A) An ERM from a patient with PDR shows prominent staining around a newly formed vessel (arrow), as well as in non-vascularised areas. (B) In an ERM from another patient with PDR, VEGF positivity is not limited to areas of neovascularisation, but is visualised in numerous other cells, including RPE cells (brown). (C) Preincubation of the primary antibody with the peptide against which it was generated (peptide control) eliminates VEGF immunostaining as seen on another section from the same ERM shown in (B).(D) VEGF staining is also demonstrated in a PVR ERM. (E) Preincubation of the primary antibody with the control peptide eliminates immunostaining in the same ERM shown in (D).(F) Most of the cells from an idiopathic ERM are also conspicuously stained for VEGF. (HistoMark Red/haematoxylin; A, B, D, E, F ×145, C ×70).
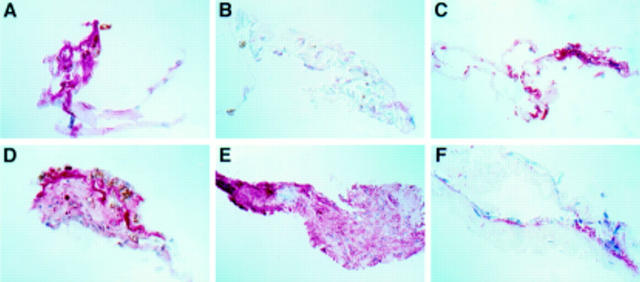
Figure 2 .
Immunostaining for multiple growth factors in a simple idiopathic ERM is visualised with a red reaction product. (A) Cellular positivity for VEGF. (B) TGF-β1 positivity (arrows) in cell processes from the same ERM. (C) Cellular staining for TGF-β2 is also demonstrated in the same ERM. (A, C: HistoMark Red/haematoxylin; B: AEC/haematoxylin; A ×145; B, C ×360).
Figure 3 .
Immunostaining for VEGF and its receptors in ERMs. (A) VEGF positivity in RPE cells containing melanin granules from a subfoveal choroidal neovascular membrane. (B) Flt-1 positivity in RPE cells containing melanin granules from a PVR ERM. (C) Double labelling for VEGF and class III β tubulin demonstrates VEGF localisation within RPE cells in a postretinal detachment ERM. VEGF is visualised with AEC and class III β tubulin with HistoMark Black. Since class III β tubulin is a marker for identifying RPE cells in ERMs, co-localisation of both colour reaction products indicates that VEGF is localised to RPE cells. (D) Nearly all of the cells in a retroretinal membrane are positive for glial fibrillary acidic protein, a marker for glial cells. (E) Another section from the same membrane illustrated in (D) showing nearly all of the cells are positive for flt-1, suggesting that glial cells express flt-1. (F) A portion of a serial section to that illustrated in (E) in which the anti-flt-1 antibodies had been preincubated with a tenfold molar excess of the peptide against which they were generated is devoid of staining, demonstrating the specificity of flt-1 staining (HistoMark Red/haematoxylin, A ,B, E, F; AEC/haematoxylin, D; A, C ×390; B, D ×155; E, F ×78).
Figure 4 .
Immunostaining for VEGF receptors in non-vascularised ERMs is visualised with a red reaction product. (A) Flt-1 staining in a PVR ERM. (B) Preincubation of the primary antibody with the peptide against which it was generated eliminates most immunostaining in the same ERM shown in (A). (C) Flt-1 positivity is demonstrated in an idiopathic ERM. (D) Flk-1 immunostaining in a subretinal membrane from a patient with PVR. (E) Flk-1 immunostaining in a PVR ERM. (F) Flk-1 immunostaining in an idiopathic ERM. (HistoMark Red/haematoxylin; A, B, C, D, F ×145, E ×70).
Figure 5 .
Immunostaining for VEGF receptors in cultured RPE cells is visualised with a red reaction product. (A) Flt-1 immunostaining is demonstrated on the surface membranes of RPE cells cultured in monolayer. (B) Preincubation of the primary antibody with the peptide against which it was generated eliminates the staining. (C) Flk-1 immunostaining is also demonstrated on the surface membranes of cultured RPE cells and is almost entirely eliminated by preincubation of the antibody with the control peptide (D). (HistoMark Red/haematoxylin, ×215).
Figure 6 .
Retinal pigmented epithelial (RPE) cells contain mRNA for the VEGF receptors, flt-1 and flk-1. Total RNA (1 µg) isolated from cultured RPE, native RPE, or retinal glia was incubated with (+) or without (−) reverse transcriptase and then run in polymerase chain reactions using the specific primers for flt-1 (A) or flk-1 (B) listed in the Methods section. Sequencing was used to confirm that the 521 bp band in A was the predicted fragment of flt-1 and the 537 bp band in B was the predicted fragment of flk-1. Cultured and native RPE contain mRNA for flt-1 and flk-1, while only flt-1 mRNA was detected in retinal glia.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamis A. P., Miller J. W., Bernal M. T., D'Amico D. J., Folkman J., Yeo T. K., Yeo K. T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994 Oct 15;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Adamis A. P., Shima D. T., Yeo K. T., Yeo T. K., Brown L. F., Berse B., D'Amore P. A., Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993 Jun 15;193(2):631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- Ahmed A., Li X. F., Dunk C., Whittle M. J., Rushton D. I., Rollason T. Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors. 1995;12(3):235–243. doi: 10.3109/08977199509036883. [DOI] [PubMed] [Google Scholar]
- Aiello L. P., Avery R. L., Arrigg P. G., Keyt B. A., Jampel H. D., Shah S. T., Pasquale L. R., Thieme H., Iwamoto M. A., Park J. E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec 1;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Aiello L. P., Pierce E. A., Foley E. D., Takagi H., Chen H., Riddle L., Ferrara N., King G. L., Smith L. E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel L. K., Raymond P. A. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990 Sep;38(9):1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- Bednarz J., Weich H. A., Rodokanaki-von Schrenck A., Engelmann K. Expression of genes coding growth factors and growth factor receptors in differentiated and dedifferentiated human corneal endothelial cells. Cornea. 1995 Jul;14(4):372–381. doi: 10.1097/00003226-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Behzadian M. A., Wang X. L., Jiang B., Caldwell R. B. Angiostatic role of astrocytes: suppression of vascular endothelial cell growth by TGF-beta and other inhibitory factor(s). Glia. 1995 Dec;15(4):480–490. doi: 10.1002/glia.440150411. [DOI] [PubMed] [Google Scholar]
- Berard M., Sordello S., Ortega N., Carrier J. L., Peyri N., Wassef M., Bertrand N., Enjolras O., Drouet L., Plouet J. Vascular endothelial growth factor confers a growth advantage in vitro and in vivo to stromal cells cultured from neonatal hemangiomas. Am J Pathol. 1997 Apr;150(4):1315–1326. [PMC free article] [PubMed] [Google Scholar]
- Campochiaro P. A., Glaser B. M. A retina-derived stimulator(s) of retinal pigment epithelial cell and astrocyte proliferation. Exp Eye Res. 1986 Sep;43(3):449–457. doi: 10.1016/s0014-4835(86)80080-x. [DOI] [PubMed] [Google Scholar]
- Campochiaro P. A., Hackett S. F., Vinores S. A., Freund J., Csaky C., LaRochelle W., Henderer J., Johnson M., Rodriguez I. R., Friedman Z. Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci. 1994 Sep;107(Pt 9):2459–2469. doi: 10.1242/jcs.107.9.2459. [DOI] [PubMed] [Google Scholar]
- Campochiaro P. A., Jerdon J. A., Glaser B. M. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis Sci. 1986 Nov;27(11):1615–1621. [PubMed] [Google Scholar]
- Charnock-Jones D. S., Sharkey A. M., Boocock C. A., Ahmed A., Plevin R., Ferrara N., Smith S. K. Vascular endothelial growth factor receptor localization and activation in human trophoblast and choriocarcinoma cells. Biol Reprod. 1994 Sep;51(3):524–530. doi: 10.1095/biolreprod51.3.524. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarkson J. G., Green W. R., Massof D. A histopathologic review of 168 cases of preretinal membrane. Am J Ophthalmol. 1977 Jul;84(1):1–17. [PubMed] [Google Scholar]
- Cohen T., Gitay-Goren H., Sharon R., Shibuya M., Halaban R., Levi B. Z., Neufeld G. VEGF121, a vascular endothelial growth factor (VEGF) isoform lacking heparin binding ability, requires cell-surface heparan sulfates for efficient binding to the VEGF receptors of human melanoma cells. J Biol Chem. 1995 May 12;270(19):11322–11326. doi: 10.1074/jbc.270.19.11322. [DOI] [PubMed] [Google Scholar]
- Cowley M., Conway B. P., Campochiaro P. A., Kaiser D., Gaskin H. Clinical risk factors for proliferative vitreoretinopathy. Arch Ophthalmol. 1989 Aug;107(8):1147–1151. doi: 10.1001/archopht.1989.01070020213027. [DOI] [PubMed] [Google Scholar]
- Frank R. N., Amin R. H., Eliott D., Puklin J. E., Abrams G. W. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996 Sep;122(3):393–403. doi: 10.1016/s0002-9394(14)72066-5. [DOI] [PubMed] [Google Scholar]
- Guerrin M., Moukadiri H., Chollet P., Moro F., Dutt K., Malecaze F., Plouët J. Vasculotropin/vascular endothelial growth factor is an autocrine growth factor for human retinal pigment epithelial cells cultured in vitro. J Cell Physiol. 1995 Aug;164(2):385–394. doi: 10.1002/jcp.1041640219. [DOI] [PubMed] [Google Scholar]
- Hanneken A., de Juan E., Jr, Lutty G. A., Fox G. M., Schiffer S., Hjelmeland L. M. Altered distribution of basic fibroblast growth factor in diabetic retinopathy. Arch Ophthalmol. 1991 Jul;109(7):1005–1011. doi: 10.1001/archopht.1991.01080070117048. [DOI] [PubMed] [Google Scholar]
- Hata Y., Nakagawa K., Ishibashi T., Inomata H., Ueno H., Sueishi K. Hypoxia-induced expression of vascular endothelial growth factor by retinal glial cells promotes in vitro angiogenesis. Virchows Arch. 1995;426(5):479–486. doi: 10.1007/BF00193171. [DOI] [PubMed] [Google Scholar]
- Hiscott P. S., Grierson I., McLeod D. Retinal pigment epithelial cells in epiretinal membranes: an immunohistochemical study. Br J Ophthalmol. 1984 Oct;68(10):708–715. doi: 10.1136/bjo.68.10.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott P. S., Grierson I., Trombetta C. J., Rahi A. H., Marshall J., McLeod D. Retinal and epiretinal glia--an immunohistochemical study. Br J Ophthalmol. 1984 Oct;68(10):698–707. doi: 10.1136/bjo.68.10.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakeman L. B., Winer J., Bennett G. L., Altar C. A., Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992 Jan;89(1):244–253. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampik A., Kenyon K. R., Michels R. G., Green W. R., de la Cruz Z. C. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol. 1981 Aug;99(8):1445–1454. doi: 10.1001/archopht.1981.03930020319025. [DOI] [PubMed] [Google Scholar]
- Kvanta A. Expression and regulation of vascular endothelial growth factor in choroidal fibroblasts. Curr Eye Res. 1995 Nov;14(11):1015–1020. doi: 10.3109/02713689508998523. [DOI] [PubMed] [Google Scholar]
- LaRochelle W. J., Robbins K. C., Aaronson S. A. Immunochemical localization of the epitope for a monoclonal antibody that neutralizes human platelet-derived growth factor mitogenic activity. Mol Cell Biol. 1989 Aug;9(8):3538–3542. doi: 10.1128/mcb.9.8.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschey K. H., Hackett S. F., Singer J. H., Campochiaro P. A. Growth factor responsiveness of human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1990 May;31(5):839–846. [PubMed] [Google Scholar]
- Lopez P. F., Sippy B. D., Lambert H. M., Thach A. B., Hinton D. R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):855–868. [PubMed] [Google Scholar]
- Lutty G. A., Merges C., Crone S., McLeod D. S. Immunohistochemical insights into sickle cell retinopathy. Curr Eye Res. 1994 Feb;13(2):125–138. doi: 10.3109/02713689409042407. [DOI] [PubMed] [Google Scholar]
- Lutty G. A., Merges C., Threlkeld A. B., Crone S., McLeod D. S. Heterogeneity in localization of isoforms of TGF-beta in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993 Mar;34(3):477–487. [PubMed] [Google Scholar]
- Malecaze F., Clamens S., Simorre-Pinatel V., Mathis A., Chollet P., Favard C., Bayard F., Plouet J. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994 Nov;112(11):1476–1482. doi: 10.1001/archopht.1994.01090230090028. [DOI] [PubMed] [Google Scholar]
- Mandriota S. J., Menoud P. A., Pepper M. S. Transforming growth factor beta 1 down-regulates vascular endothelial growth factor receptor 2/flk-1 expression in vascular endothelial cells. J Biol Chem. 1996 May 10;271(19):11500–11505. doi: 10.1074/jbc.271.19.11500. [DOI] [PubMed] [Google Scholar]
- Miller J. W., Adamis A. P., Shima D. T., D'Amore P. A., Moulton R. S., O'Reilly M. S., Folkman J., Dvorak H. F., Brown L. F., Berse B. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994 Sep;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- Mudhar H. S., Pollock R. A., Wang C., Stiles C. D., Richardson W. D. PDGF and its receptors in the developing rodent retina and optic nerve. Development. 1993 Jun;118(2):539–552. doi: 10.1242/dev.118.2.539. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Hayashi H., Vinores S. A., Moromizato Y., Campochiaro P. A., Oshima K. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997 Apr;64(4):505–517. doi: 10.1006/exer.1996.0239. [DOI] [PubMed] [Google Scholar]
- Pe'er J., Folberg R., Itin A., Gnessin H., Hemo I., Keshet E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol. 1996 Mar;80(3):241–245. doi: 10.1136/bjo.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe'er J., Shweiki D., Itin A., Hemo I., Gnessin H., Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995 Jun;72(6):638–645. [PubMed] [Google Scholar]
- Pierce E. A., Avery R. L., Foley E. D., Aiello L. P., Smith L. E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Pournaras C. J., Tsacopoulos M., Strommer K., Gilodi N., Leuenberger P. M. Scatter photocoagulation restores tissue hypoxia in experimental vasoproliferative microangiopathy in miniature pigs. Ophthalmology. 1990 Oct;97(10):1329–1333. doi: 10.1016/s0161-6420(90)32414-4. [DOI] [PubMed] [Google Scholar]
- Quinn T. P., Peters K. G., De Vries C., Ferrara N., Williams L. T. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Reilly T. M., Taylor D. S., Herblin W. F., Thoolen M. J., Chiu A. T., Watson D. W., Timmermans P. B. Monoclonal antibodies directed against basic fibroblast growth factor which inhibit its biological activity in vitro and in vivo. Biochem Biophys Res Commun. 1989 Oct 31;164(2):736–743. doi: 10.1016/0006-291x(89)91521-0. [DOI] [PubMed] [Google Scholar]
- Robbins S. G., Mixon R. N., Wilson D. J., Hart C. E., Robertson J. E., Westra I., Planck S. R., Rosenbaum J. T. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Invest Ophthalmol Vis Sci. 1994 Sep;35(10):3649–3663. [PubMed] [Google Scholar]
- Sen H. A., Campochiaro P. A. Intravitreous injection of adenosine or its agonists causes breakdown of the blood-retinal barrier. Arch Ophthalmol. 1989 Sep;107(9):1364–1367. doi: 10.1001/archopht.1989.01070020434049. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Sivalingam A., Kenney J., Brown G. C., Benson W. E., Donoso L. Basic fibroblast growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1990 Jun;108(6):869–872. doi: 10.1001/archopht.1990.01070080113046. [DOI] [PubMed] [Google Scholar]
- Starksen N. F., Harsh G. R., 4th, Gibbs V. C., Williams L. T. Regulated expression of the platelet-derived growth factor A chain gene in microvascular endothelial cells. J Biol Chem. 1987 Oct 25;262(30):14381–14384. [PubMed] [Google Scholar]
- Takahashi T., Shirasawa T., Miyake K., Yahagi Y., Maruyama N., Kasahara N., Kawamura T., Matsumura O., Mitarai T., Sakai O. Protein tyrosine kinases expressed in glomeruli and cultured glomerular cells: Flt-1 and VEGF expression in renal mesangial cells. Biochem Biophys Res Commun. 1995 Apr 6;209(1):218–226. doi: 10.1006/bbrc.1995.1492. [DOI] [PubMed] [Google Scholar]
- Terman B. I., Dougher-Vermazen M., Carrion M. E., Dimitrov D., Armellino D. C., Gospodarowicz D., Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Tolentino M. J., Miller J. W., Gragoudas E. S., Jakobiec F. A., Flynn E., Chatzistefanou K., Ferrara N., Adamis A. P. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996 Nov;103(11):1820–1828. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Campochiaro P. A., Conway B. P. Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci. 1990 Jan;31(1):14–28. [PubMed] [Google Scholar]
- Vinores S. A., Campochiaro P. A., McGehee R., Orman W., Hackett S. F., Hjelmeland L. M. Ultrastructural and immunocytochemical changes in retinal pigment epithelium, retinal glia, and fibroblasts in vitreous culture. Invest Ophthalmol Vis Sci. 1990 Dec;31(12):2529–2545. [PubMed] [Google Scholar]
- Vinores S. A., Derevjanik N. L., Mahlow J., Hackett S. F., Haller J. A., deJuan E., Frankfurter A., Campochiaro P. A. Class III beta-tubulin in human retinal pigment epithelial cells in culture and in epiretinal membranes. Exp Eye Res. 1995 Apr;60(4):385–400. doi: 10.1016/s0014-4835(05)80095-8. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Henderer J. D., Mahlow J., Chiu C., Derevjanik N. L., Larochelle W., Csaky C., Campochiaro P. A. Isoforms of platelet-derived growth factor and its receptors in epiretinal membranes: immunolocalization to retinal pigmented epithelial cells. Exp Eye Res. 1995 Jun;60(6):607–619. doi: 10.1016/s0014-4835(05)80003-x. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Herman M. M., Hackett S. F., Campochiaro P. A. A morphological and immunohistochemical study of human retinal pigment epithelial cells, retinal glia, and fibroblasts grown on Gelfoam matrix in an organ culture system. A comparison of structural and nonstructural proteins and their application to cell type identification. Graefes Arch Clin Exp Ophthalmol. 1993 May;231(5):279–288. doi: 10.1007/BF00919106. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Küchle M., Mahlow J., Chiu C., Green W. R., Campochiaro P. A. Blood-ocular barrier breakdown in eyes with ocular melanoma. A potential role for vascular endothelial growth factor/vascular permeability factor. Am J Pathol. 1995 Nov;147(5):1289–1297. [PMC free article] [PubMed] [Google Scholar]
- Vinores S. A., Youssri A. I., Luna J. D., Chen Y. S., Bhargave S., Vinores M. A., Schoenfeld C. L., Peng B., Chan C. C., LaRochelle W. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997 Jan;12(1):99–109. [PubMed] [Google Scholar]
- Yang X., Cepko C. L. Flk-1, a receptor for vascular endothelial growth factor (VEGF), is expressed by retinal progenitor cells. J Neurosci. 1996 Oct 1;16(19):6089–6099. doi: 10.1523/JNEUROSCI.16-19-06089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries C., Escobedo J. A., Ueno H., Houck K., Ferrara N., Williams L. T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992 Feb 21;255(5047):989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]