Full Text
The Full Text of this article is available as a PDF (259.6 KB).
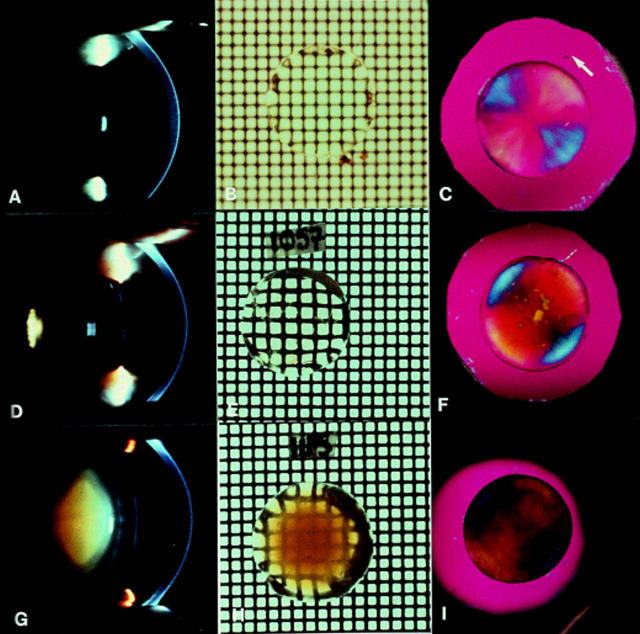
Figure 1 .
Images of normal human lenses (A, B, and C), posterior polar cataract (D, E, F), and pure nuclear cataract (G, H, I). Note that slit lamp camera images (A, D, G) all have a scattering reflect artefact (small white rectangle). The normal subject (A) was 40 years of age and the accompanying in vitro grid photographs (B) and polarising images (C) were obtained from a donor eye of similar age (42 years). The white arrow gives the direction of plane of polarisation of the major axis of the first order red plate.63 Note that (B) was photographed under fluid and so the grid is out of focus compared with (E) and (H), which were photographed in air. Also note that the polarising images of the cataractous lenses (F and I) maintain the blue/yellow radially symmetrical pattern of the normal lens (C) except where the opacities occur in highly localised polar cataract (F) and where the brunescence is strongest in the nuclear cataract (I). The images (D), (E), (G), (H) are taken from Marcantonio et al11 while the additional images are unpublished.
Figure 2 .

Brewster's model of the arrangement of fibre cells within the lens.15 He discovered that the fibre diameter varied throughout the lens and that the tips met in a series of suture lines on the optic axis. Hence, when viewed along the optic axis (as in Fig 1, C, F, and I), such an arrangement would give rise to positive birefringence such that the fibres lying along the direction of the first order red plate give a blue addition colour and those at right angles, a yellow subtraction colour.14 15 63
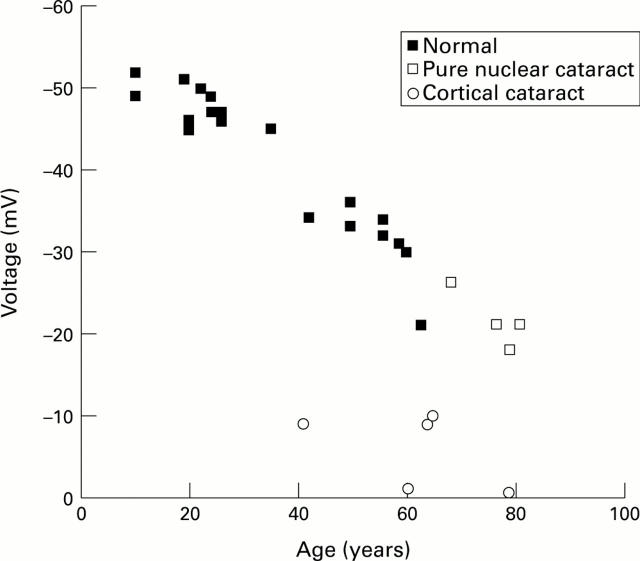
Figure 3 .
Human lens membrane voltage measured in eye bank and cataract lenses. Note that the pure nuclear cataracts follow the pattern of normal lenses, while the cortical cataracts have voltages considerably lower than normal lenses of a similar age. Data taken from Duncan and Hightower43 and Duncan et al.45
Figure 4 .
Relative cation permeability (PNa/PK) of human lenses as a function of age computed from measurements of ion concentration and electrical potential.45 The solid line shows the change with age of the mean optical density (OD) of the human lens measured at 490 nm.19
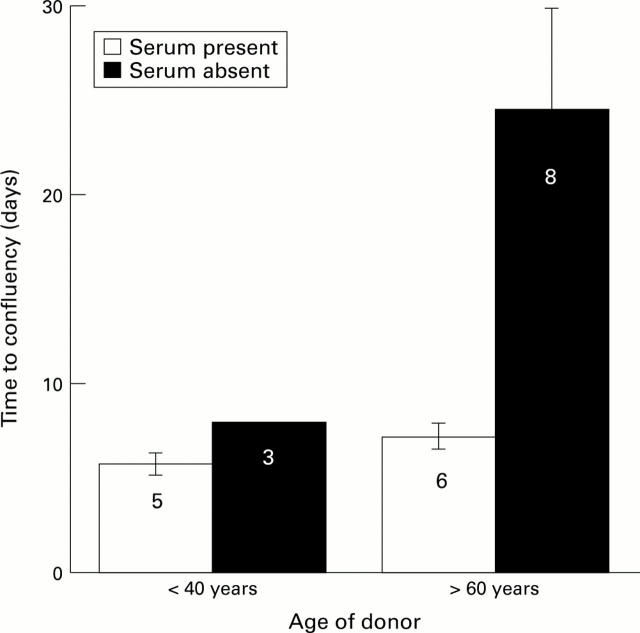
Figure 5 .
The effects of age and serum on time to confluence of cells on the human posterior capsule. Cell growth was observed using phase contrast microscopy. Confluency was taken at the point when 100% of the area on the posterior capsule, within the confines of the capsulorhexis, was covered by epithelial cells. Serum data were taken from Liu et al58 and serum free data from Wormstone et al.17 The numbers of different capsules used to obtain the data are given.
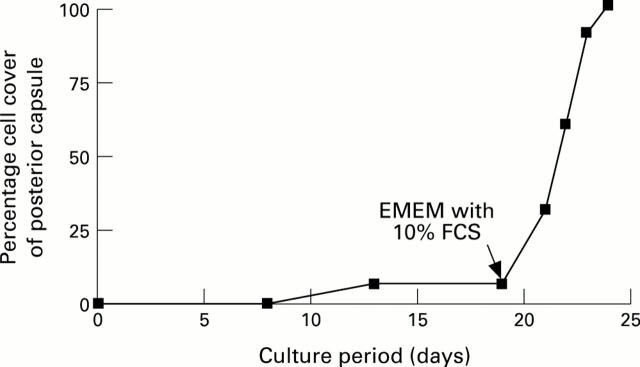
Figure 6 .
Cell cover on the posterior capsule from a 78-year-old donor lens as a function of time. The capsular bag was initially cultured in protein free medium where it ceased to grow after 12 days. The culture medium was then supplemented with serum after 19 days. The time to confluency (100%) was estimated as in Figure 5. Data taken from Wormstone et al.17
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apple D. J., Solomon K. D., Tetz M. R., Assia E. I., Holland E. Y., Legler U. F., Tsai J. C., Castaneda V. E., Hoggatt J. P., Kostick A. M. Posterior capsule opacification. Surv Ophthalmol. 1992 Sep-Oct;37(2):73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A., Ansari N. H., Wang L., Khanna P., Wang C., Srivastava S. K. Calcium-mediated disintegrative globulization of isolated ocular lens fibers mimics cataractogenesis. Exp Eye Res. 1995 Sep;61(3):303–310. doi: 10.1016/s0014-4835(05)80125-3. [DOI] [PubMed] [Google Scholar]
- Brown N., Hungerford J. The influence of the size of the lens in ocular disease. Trans Ophthalmol Soc U K. 1982;102(Pt 3):359–363. [PubMed] [Google Scholar]
- Brown N. The change in shape and internal form of the lens of the eye on accommodation. Exp Eye Res. 1973 Apr;15(4):441–459. doi: 10.1016/0014-4835(73)90136-x. [DOI] [PubMed] [Google Scholar]
- Cumming R. G., Mitchell P. Hormone replacement therapy, reproductive factors, and cataract. The Blue Mountains Eye Study. Am J Epidemiol. 1997 Feb 1;145(3):242–249. doi: 10.1093/oxfordjournals.aje.a009097. [DOI] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Donner K., Hemilä S., Kalamkarov G., Koskelainen A., Pogozheva I., Rebrik T. Sulfhydryl binding reagents increase the conductivity of the light-sensitive channel and inhibit phototransduction in retinal rods. Exp Eye Res. 1990 Jul;51(1):97–105. doi: 10.1016/0014-4835(90)90176-u. [DOI] [PubMed] [Google Scholar]
- Duncan G., Hightower K. R., Gandolfi S. A., Tomlinson J., Maraini G. Human lens membrane cation permeability increases with age. Invest Ophthalmol Vis Sci. 1989 Aug;30(8):1855–1859. [PubMed] [Google Scholar]
- Duncan G., Jacob T. J. Calcium and the physiology of cataract. Ciba Found Symp. 1984;106:132–152. doi: 10.1002/9780470720875.ch8. [DOI] [PubMed] [Google Scholar]
- Frezzotti R., Caporossi A., Mastrangelo D., Hadjistilianou T., Tosi P., Cintorino M., Minacci C. Pathogenesis of posterior capsular opacification. Part II: Histopathological and in vitro culture findings. J Cataract Refract Surg. 1990 May;16(3):353–360. doi: 10.1016/s0886-3350(13)80708-0. [DOI] [PubMed] [Google Scholar]
- Gandolfi S. A., Duncan G., Tomlinson J., Maraini G. Mammalian lens inter-fiber resistance is modulated by calcium and calmodulin. Curr Eye Res. 1990 Jun;9(6):533–541. doi: 10.3109/02713689008999593. [DOI] [PubMed] [Google Scholar]
- Griffing G. T., Allen S. H. Estrogen replacement therapy at menopause. How benefits outweigh risks. Postgrad Med. 1994 Oct;96(5):131–140. [PubMed] [Google Scholar]
- Hales A. M., Chamberlain C. G., McAvoy J. W. Cataract induction in lenses cultured with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1995 Jul;36(8):1709–1713. [PubMed] [Google Scholar]
- Hales A. M., Chamberlain C. G., Murphy C. R., McAvoy J. W. Estrogen protects lenses against cataract induced by transforming growth factor-beta (TGFbeta). J Exp Med. 1997 Jan 20;185(2):273–280. doi: 10.1084/jem.185.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales A. M., Schulz M. W., Chamberlain C. G., McAvoy J. W. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994 Dec;13(12):885–890. doi: 10.3109/02713689409015091. [DOI] [PubMed] [Google Scholar]
- Hankinson S. E., Stampfer M. J., Seddon J. M., Colditz G. A., Rosner B., Speizer F. E., Willett W. C. Nutrient intake and cataract extraction in women: a prospective study. BMJ. 1992 Aug 8;305(6849):335–339. doi: 10.1136/bmj.305.6849.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Chylack L. T., Jr, Susan S. R., Lo W. K., Bobrowski W. F. Elemental and ultrastructural analysis of specific human lens opacities. Invest Ophthalmol Vis Sci. 1982 Jul;23(1):1–13. [PubMed] [Google Scholar]
- Harding J. J., Rixon K. C., Marriott F. H. Men have heavier lenses than women of the same age. Exp Eye Res. 1977 Dec;25(6):651–651. doi: 10.1016/0014-4835(77)90143-9. [DOI] [PubMed] [Google Scholar]
- Hockwin O., Dragomirescu V., Laser H. Measurements of lens transparency or its disturbances by densitometric image analysis of Scheimpflug photographs. Graefes Arch Clin Exp Ophthalmol. 1982;219(6):255–262. doi: 10.1007/BF00231409. [DOI] [PubMed] [Google Scholar]
- Ibaraki N., Ohara K., Miyamoto T. Membranous outgrowth suggesting lens epithelial cell proliferation in pseudophakic eyes. Am J Ophthalmol. 1995 Jun;119(6):706–711. doi: 10.1016/s0002-9394(14)72774-6. [DOI] [PubMed] [Google Scholar]
- Jacob T. J., Bangham J. A., Duncan G. Characterization of a cation channel on the apical surface of the frog lens epithelium. Q J Exp Physiol. 1985 Jul;70(3):403–421. doi: 10.1113/expphysiol.1985.sp002925. [DOI] [PubMed] [Google Scholar]
- Jones N. P., McLeod D., Boulton M. E. Massive proliferation of lens epithelial remnants after Nd-YAG laser capsulotomy. Br J Ophthalmol. 1995 Mar;79(3):261–263. doi: 10.1136/bjo.79.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn H. A., Leibowitz H. M., Ganley J. P., Kini M. M., Colton T., Nickerson R. S., Dawber T. R. The Framingham Eye Study. I. Outline and major prevalence findings. Am J Epidemiol. 1977 Jul;106(1):17–32. doi: 10.1093/oxfordjournals.aje.a112428. [DOI] [PubMed] [Google Scholar]
- Liu C. S., Wormstone I. M., Duncan G., Marcantonio J. M., Webb S. F., Davies P. D. A study of human lens cell growth in vitro. A model for posterior capsule opacification. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):906–914. [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- Lucas V. A., Duncan G., Davies P. Membrane permeability characteristics of perfused human senile cataractous lenses. Exp Eye Res. 1986 Feb;42(2):151–165. doi: 10.1016/0014-4835(86)90039-4. [DOI] [PubMed] [Google Scholar]
- Marcantonio J. M., Duncan G. Amino acid transport and crystallin synthesis in the bovine lens. Exp Eye Res. 1983 Mar;36(3):429–440. doi: 10.1016/0014-4835(83)90124-0. [DOI] [PubMed] [Google Scholar]
- Marcantonio J. M., Duncan G., Davies P. D., Bushell A. R. Classification of human senile cataracts by nuclear colour and sodium content. Exp Eye Res. 1980 Aug;31(2):227–237. doi: 10.1016/0014-4835(80)90080-9. [DOI] [PubMed] [Google Scholar]
- Marcantonio J. M., Duncan G., Rink H. Calcium-induced opacification and loss of protein in the organ-cultured bovine lens. Exp Eye Res. 1986 Jun;42(6):617–630. doi: 10.1016/0014-4835(86)90051-5. [DOI] [PubMed] [Google Scholar]
- McAvoy J. W., Chamberlain C. G. Growth factors in the eye. Prog Growth Factor Res. 1990;2(1):29–43. doi: 10.1016/0955-2235(90)90008-8. [DOI] [PubMed] [Google Scholar]
- Moisseiev J., Bartov E., Schochat A., Blumenthal M. Long-term study of the prevalence of capsular opacification following extracapsular cataract extraction. J Cataract Refract Surg. 1989 Sep;15(5):531–533. doi: 10.1016/s0886-3350(89)80110-5. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Fukui H. N., Kinoshita J. H. Differential metabolism and leakage of protein in an inherited cataract and a normal lens cultured with ouabain. Nature. 1978 Aug 10;274(5671):558–562. doi: 10.1038/274558a0. [DOI] [PubMed] [Google Scholar]
- Rae J. L., Rae J. S. Whole-cell currents from noncultured human lens epithelium. Invest Ophthalmol Vis Sci. 1992 Jun;33(7):2262–2268. [PubMed] [Google Scholar]
- Rakic J. M., Galand A., Vrensen G. F. Separation of fibres from the capsule enhances mitotic activity of human lens epithelium. Exp Eye Res. 1997 Jan;64(1):67–72. doi: 10.1006/exer.1996.0179. [DOI] [PubMed] [Google Scholar]
- Sanderson J., Duncan G. pCMPS-induced changes in lens membrane permeability and transparency. Invest Ophthalmol Vis Sci. 1993 Jul;34(8):2518–2525. [PubMed] [Google Scholar]
- Schmitt-Gräff A., Pau H., Spahr R., Piper H. M., Skalli O., Gabbiani G. Appearance of alpha-smooth muscle actin in human eye lens cells of anterior capsular cataract and in cultured bovine lens-forming cells. Differentiation. 1990 Apr;43(2):115–122. doi: 10.1111/j.1432-0436.1990.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Shaffer R. N., Hetherington J., Jr Anticholinesterase drugs and cataracts. Am J Ophthalmol. 1966 Oct;62(4):613–618. doi: 10.1016/0002-9394(66)92181-7. [DOI] [PubMed] [Google Scholar]
- Shah S. M., Spalton D. J., Muir M. K. Specular microscopy of the anterior intraocular lens surface. Eye (Lond) 1993;7(Pt 5):707–710. doi: 10.1038/eye.1993.161. [DOI] [PubMed] [Google Scholar]
- Thomas G. R., Williams M. B., Sanderson J., Duncan G. The human lens possesses acetylcholine receptors that are functional throughout life. Exp Eye Res. 1997 May;64(5):849–852. doi: 10.1006/exer.1996.0243. [DOI] [PubMed] [Google Scholar]
- Truscott R. J., Marcantonio J. M., Tomlinson J., Duncan G. Calcium-induced opacification and proteolysis in the intact rat lens. Invest Ophthalmol Vis Sci. 1990 Nov;31(11):2405–2411. [PubMed] [Google Scholar]
- Vrensen G. F., Willekens B., De Jong P. T., Shun-Shin G. A., Brown N. P., Bron A. J. Heterogeneity in ultrastructure and elemental composition of perinuclear lens retrodots. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):199–206. [PubMed] [Google Scholar]
- Vrensen G., Willekens B. Classification and prevalence of early senile lens opacities in human donor eyes. Dev Ophthalmol. 1989;17:181–187. doi: 10.1159/000417026. [DOI] [PubMed] [Google Scholar]
- Wang L., Bhatnagar A., Ansari N. H., Dhir P., Srivastava S. K. Mechanism of calcium-induced disintegrative globulization of rat lens fiber cells. Invest Ophthalmol Vis Sci. 1996 Apr;37(5):915–922. [PubMed] [Google Scholar]
- Weale R. A. Sex, age and the birefringence of the human crystalline lens. Exp Eye Res. 1979 Oct;29(4):449–461. doi: 10.1016/0014-4835(79)90062-9. [DOI] [PubMed] [Google Scholar]
- Williams M. R., Duncan G., Riach R. A., Webb S. F. Acetylcholine receptors are coupled to mobilization of intracellular calcium cultured human lens cells. Exp Eye Res. 1993 Sep;57(3):381–384. doi: 10.1006/exer.1993.1138. [DOI] [PubMed] [Google Scholar]
- Wormstone I. M., Liu C. S., Rakic J. M., Marcantonio J. M., Vrensen G. F., Duncan G. Human lens epithelial cell proliferation in a protein-free medium. Invest Ophthalmol Vis Sci. 1997 Feb;38(2):396–404. [PubMed] [Google Scholar]