Abstract
BACKGROUND—Lipofuscin granules in the retinal pigment epithelium are lipid protein aggregates which are thought to represent the lifelong accumulation of the non-degradable end products from the phagocytosis of photoreceptor outer segments. Given the increasing evidence for a key role for vitamin A in the formation of ocular lipofuscin, the fluorophores generated by reacting vitamin A with lipid were assessed. METHODS—Reaction mixtures consisting of vitamin A (retinol) or its aldehyde (retinal) and (a) isolated rod outer segments, (b) the lipid extract of rod outer segments, (c) protein, or (d) liposomes were incubated at either pH 4.5 or 7.0 for up to 42 days. The fluorescence characteristics and mobility of the chloroform soluble fluorophores generated were compared with those extracted from purified human lipofuscin. Finally, the effect of lysosomal degradation on fluorophores generated in the above mixtures was assessed. RESULTS—Major spectral changes were observed when ROS or liposomes were incubated with retinal. These changes were pH dependent and did not occur if retinal was replaced with retinol. A number of the fluorophores generated exhibited similar fluorescence characteristics and chromatographic mobility to those of lipofuscin. Neither the presence of protein nor exposure to lysosomal enzymes had any effect on the spectral profile or fluorophore mobility of the fluorophores generated. CONCLUSIONS—These results suggest that some of the chloroform soluble fluorophores of lipofuscin are formed as a direct reaction product of retinal and lipid.
Full Text
The Full Text of this article is available as a PDF (164.2 KB).
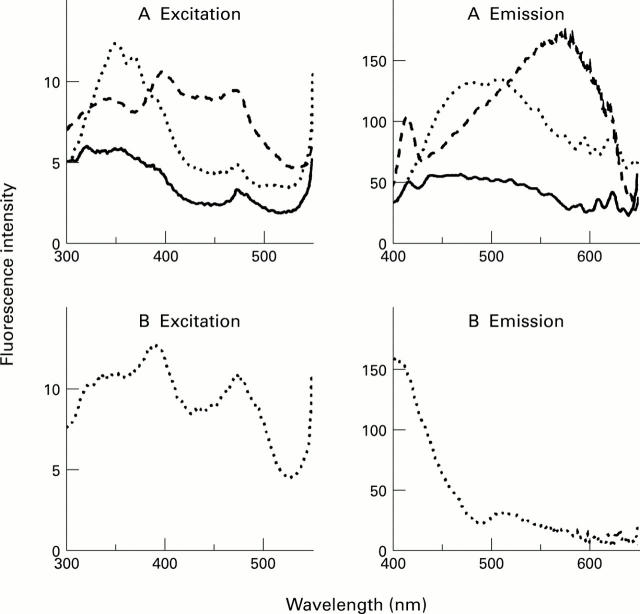
Figure 1 .
Spectral profile of fluorophores formed by incubating retinal with ROS. Retinal incubated with ROS at pH 7 produced long wavelength emitting fluorophores (A); no such fluorophores were formed at pH 4.5 (B). Spectra shown are of freshly prepared (—) and incubated ( . . .) mixtures of retinal and ROS and are representative of a typical experiment. Similar spectral profiles were obtained in the presence of BSA. A typical spectral profile of lipofuscin isolated from five 60 to 69 year old donors (- -) is shown for comparison.
Figure 2 .
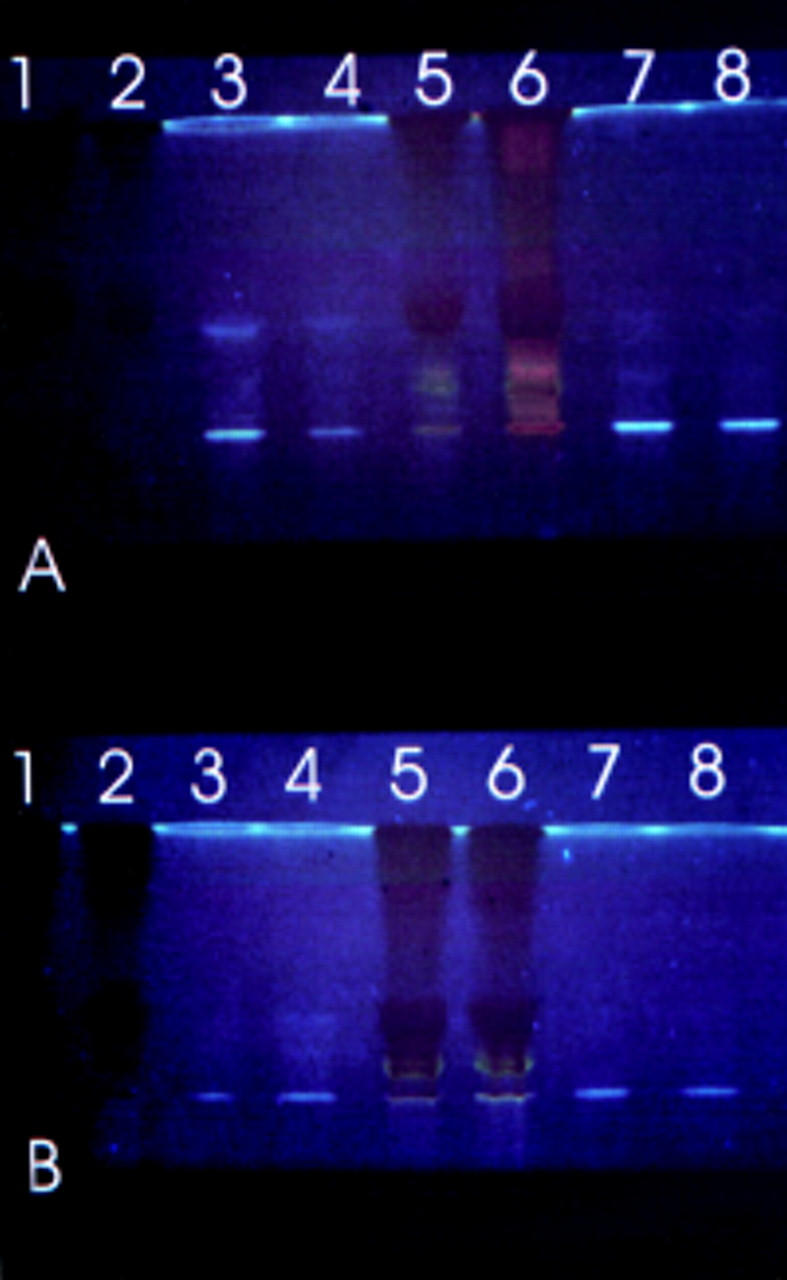
TLC profile of chloroform soluble fluorophores generated by incubating retinal with ROS. Lanes 1 to 4 contain freshly prepared material; lanes 5 to 8 material after 7 days' incubation. Plate (A) contains samples at pH 7, plate (B) samples at pH 4.5. Lanes 1 and 5, retinal + ROS + BSA; lanes 2 and 6, retinal + ROS; lanes 3, 4, 7, and 8, ROS alone. The plates shown are representative of a typical experiment.
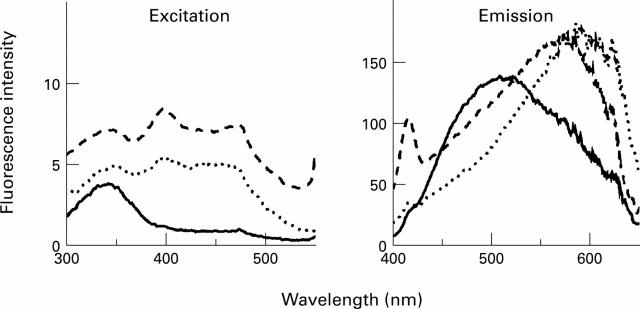
Figure 3 .
Spectral profile of fluorophores formed by incubating retinal with liposomes. Spectra shown are of a freshly prepared (—) and incubated ( . . .) retinal/liposome mixture and are representative of a typical experiment. A similar profile was produced in the presence of BSA. A typical spectral profile of lipofuscin isolated from five 60 to 69 year old donors (- -) is shown for comparison.
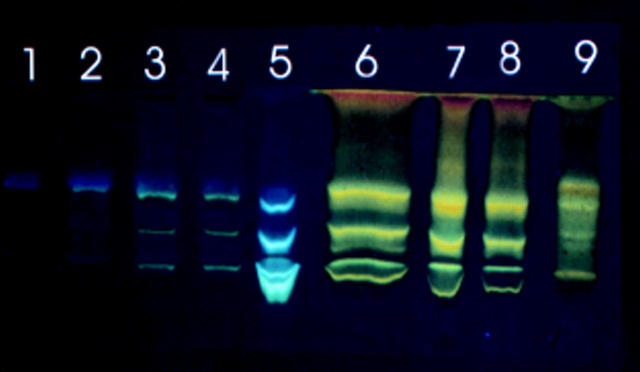
Figure 4 .
TLC profile of chloroform soluble fluorophores generated by incubating liposomes with retinal. Lanes 1 to 4 contain freshly prepared material; lanes 5 to 8 material after 7 days' incubation. Lanes 1 and 5, liposomes; lanes 2 and 6, liposomes + retinal; lanes 3, 4, 7, and 8, liposomes + retinal + BSA; lane 9, lipofuscin. The plate is representative of a typical experiment.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch E., Horwitz J., Bok D. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem. 1993 Feb;41(2):253–263. doi: 10.1177/41.2.8419462. [DOI] [PubMed] [Google Scholar]
- Boulton M., Docchio F., Dayhaw-Barker P., Ramponi R., Cubeddu R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res. 1990;30(9):1291–1303. doi: 10.1016/0042-6989(90)90003-4. [DOI] [PubMed] [Google Scholar]
- Boulton M., McKechnie N. M., Breda J., Bayly M., Marshall J. The formation of autofluorescent granules in cultured human RPE. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):82–89. [PubMed] [Google Scholar]
- Boulton M., Moriarty P., Jarvis-Evans J., Marcyniuk B. Regional variation and age-related changes of lysosomal enzymes in the human retinal pigment epithelium. Br J Ophthalmol. 1994 Feb;78(2):125–129. doi: 10.1136/bjo.78.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral L., Unger W., Boulton M., Marshall J. A microsystem to assay lysosomal enzyme activities in cultured retinal pigment epithelial cells. Curr Eye Res. 1988 Nov;7(11):1097–1104. doi: 10.3109/02713688809001880. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Synthesis and characterization of the fluorescent products derived from malonaldehyde and amino acids. Biochemistry. 1969 Jul;8(7):2821–2826. doi: 10.1021/bi00835a019. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delori F. C., Dorey C. K., Staurenghi G., Arend O., Goger D. G., Weiter J. J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995 Mar;36(3):718–729. [PubMed] [Google Scholar]
- Docchio F., Boulton M., Cubeddu R., Ramponi R., Barker P. D. Age-related changes in the fluorescence of melanin and lipofuscin granules of the retinal pigment epithelium: a time-resolved fluorescence spectroscopy study. Photochem Photobiol. 1991 Aug;54(2):247–253. doi: 10.1111/j.1751-1097.1991.tb02013.x. [DOI] [PubMed] [Google Scholar]
- Eldred G. E., Katz M. L. Fluorophores of the human retinal pigment epithelium: separation and spectral characterization. Exp Eye Res. 1988 Jul;47(1):71–86. doi: 10.1016/0014-4835(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Eldred G. E., Katz M. L. The autofluorescent products of lipid peroxidation may not be lipofuscin-like. Free Radic Biol Med. 1989;7(2):157–163. doi: 10.1016/0891-5849(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Eldred G. E., Lasky M. R. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993 Feb 25;361(6414):724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- Eldred G. E., Miller G. V., Stark W. S., Feeney-Burns L. Lipofuscin: resolution of discrepant fluorescence data. Science. 1982 May 14;216(4547):757–759. doi: 10.1126/science.7079738. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Feeney-Burns L., Eldred G. E. The fate of the phagosome: conversion to 'age pigment' and impact in human retinal pigment epithelium. Trans Ophthalmol Soc U K. 1983;103(Pt 4):416–421. [PubMed] [Google Scholar]
- Feeney-Burns L., Hilderbrand E. S., Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984 Feb;25(2):195–200. [PubMed] [Google Scholar]
- Fliesler S. J., Anderson R. E. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22(2):79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Christianson J. S., Gao C. L., Handelman G. J. Iron-induced fluorescence in the retina: dependence on vitamin A. Invest Ophthalmol Vis Sci. 1994 Sep;35(10):3613–3624. [PubMed] [Google Scholar]
- Katz M. L., Drea C. M., Eldred G. E., Hess H. H., Robison W. G., Jr Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp Eye Res. 1986 Oct;43(4):561–573. doi: 10.1016/s0014-4835(86)80023-9. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Drea C. M., Robison W. G., Jr Relationship between dietary retinol and lipofuscin in the retinal pigment epithelium. Mech Ageing Dev. 1986 Aug;35(3):291–305. doi: 10.1016/0047-6374(86)90131-4. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Gao C. L., Rice L. M. Formation of lipofuscin-like fluorophores by reaction of retinal with photoreceptor outer segments and liposomes. Mech Ageing Dev. 1996 Dec 20;92(2-3):159–174. doi: 10.1016/s0047-6374(96)01817-9. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Norberg M. Influence of dietary vitamin A on autofluorescence of leupeptin-induced inclusions in the retinal pigment epithelium. Exp Eye Res. 1992 Feb;54(2):239–246. doi: 10.1016/s0014-4835(05)80213-1. [DOI] [PubMed] [Google Scholar]
- Rózanowska M., Jarvis-Evans J., Korytowski W., Boulton M. E., Burke J. M., Sarna T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995 Aug 11;270(32):18825–18830. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- Wyszynski R. E., Bruner W. E., Cano D. B., Morgan K. M., Davis C. B., Sternberg P. A donor-age-dependent change in the activity of alpha-mannosidase in human cultured RPE cells. Invest Ophthalmol Vis Sci. 1989 Nov;30(11):2341–2347. [PubMed] [Google Scholar]
- Yin D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic Biol Med. 1996;21(6):871–888. doi: 10.1016/0891-5849(96)00175-x. [DOI] [PubMed] [Google Scholar]
- Yin D., Yuan X., Brunk U. T. Test-tube simulated lipofuscinogenesis. Effect of oxidative stress on autophagocytotic degradation. Mech Ageing Dev. 1995 Jun 30;81(1):37–50. doi: 10.1016/0047-6374(94)01580-f. [DOI] [PubMed] [Google Scholar]
- Young R. W. Solar radiation and age-related macular degeneration. Surv Ophthalmol. 1988 Jan-Feb;32(4):252–269. doi: 10.1016/0039-6257(88)90174-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman W. F., Godchaux W., 3rd, Belkin M. The relative proportions of lysosomal enzyme activities in bovine retinal pigment epithelium. Exp Eye Res. 1983 Jan;36(1):151–158. doi: 10.1016/0014-4835(83)90098-2. [DOI] [PubMed] [Google Scholar]