Abstract
BACKGROUND—The level of HLA expression on a tumour may influence the immunological response against this tumour, and vice versa. HLA expression was determined in a primary uveal melanoma, its metastases, and on a cell line derived from this melanoma, and the presence and type of infiltrate in tissue sections were also studied. METHODS—Immunohistochemistry with monoclonal antibodies (MAbs) against HLA class I and II, T cells, NK cells, and macrophages. RESULTS—Primary and metastatic lesions, as well as the cell line showed high levels of expression of the monomorphic determinants of HLA class I. Expression of the polymorphic HLA-A2 and HLA-A3 antigens was decreased on metastases to the skin and liver. HLA-Bw4 expression was low on all lesions, as well as expression of HLA class II. Tumour infiltrating cells consisted mainly of CD3, CD4, and CD8 positive cells. Expression on the cell line corresponded to expression on the primary tumour. CONCLUSION—The primary uveal melanoma as well as the cell line showed a high expression of monomorphic and polymorphic HLA-A antigens, while metastases showed a high expression of monomorphic and a lower expression of polymorphic antigens. This variation in expression may support tumour cell escape from NK cells as well as CTL mediated lysis.
Full Text
The Full Text of this article is available as a PDF (161.5 KB).
Figure 1 .
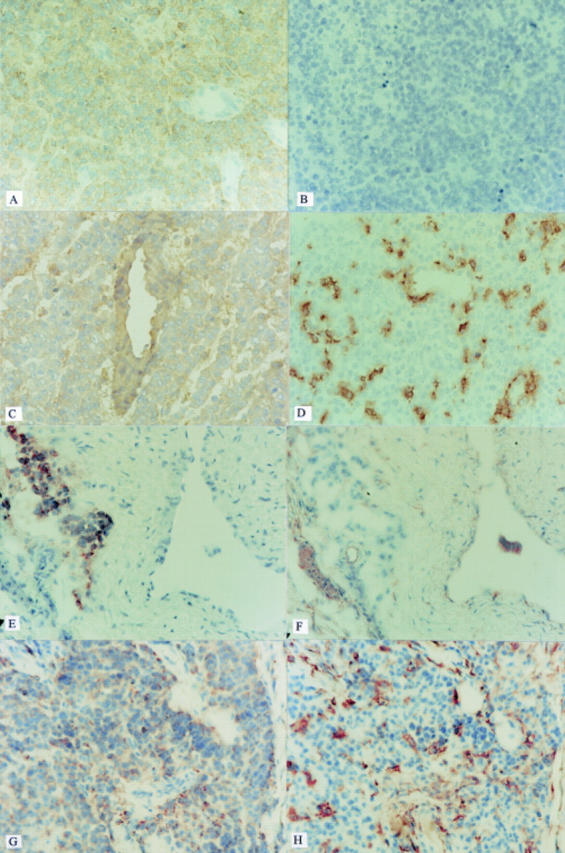
Expression of: (A) NKI-beteb on part one of the primary tumour. All tumour cells stain positively, while vessels are not stained; (B) negative control (PBS) on part one of the primary tumour. No staining is observed; (C) HLA-A2 on part one of the primary tumour. Tumour cells, as well as vessels stain positive; (D) HLA-DR on part one of the primary tumour. Scattered infiltrating cells and part of the tumour cells stain positively; (E) NKI-beteb on the liver metastasis. A cluster of positive staining cells can be discerned near a negative vessel; (F) HLA-A2 on the liver metastasis. Consecutive section to (E) the same cluster of NKI-beteb positive tumour cells does not stain positive for HLA-A2; (G) NKI-beteb on the skin metastasis. Virtually the whole section stains positive, excluding the vessels; (H) HLA-DR on the skin metastasis. Consecutive section to (G) scattered infiltrating cells and part of the tumour cells stain positively. (Original magnification ×200.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Concha A., Esteban F., Cabrera T., Ruiz-Cabello F., Garrido F. Tumor aggressiveness and MHC class I and II antigens in laryngeal and breast cancer. Semin Cancer Biol. 1991 Feb;2(1):47–54. [PubMed] [Google Scholar]
- De Waard-Siebinga I., Blom D. J., Griffioen M., Schrier P. I., Hoogendoorn E., Beverstock G., Danen E. H., Jager M. J. Establishment and characterization of an uveal-melanoma cell line. Int J Cancer. 1995 Jul 17;62(2):155–161. doi: 10.1002/ijc.2910620208. [DOI] [PubMed] [Google Scholar]
- Ferrone S., Marincola F. M. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995 Oct;16(10):487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- Garrido F., Cabrera T., Concha A., Glew S., Ruiz-Cabello F., Stern P. L. Natural history of HLA expression during tumour development. Immunol Today. 1993 Oct;14(10):491–499. doi: 10.1016/0167-5699(93)90264-L. [DOI] [PubMed] [Google Scholar]
- Gumperz J. E., Parham P. The enigma of the natural killer cell. Nature. 1995 Nov 16;378(6554):245–248. doi: 10.1038/378245a0. [DOI] [PubMed] [Google Scholar]
- Jager M. J., de Wolff-Rouendaal D., Breebaart A. C., Ruiter D. J. Expression of HLA antigens in paraffin sections of uveal melanomas. Doc Ophthalmol. 1986 Dec 30;64(1):69–76. doi: 10.1007/BF00166687. [DOI] [PubMed] [Google Scholar]
- Jager M. J., van der Pol J. P., de Wolff-Rouendaal D., de Jong P. T., Ruiter D. J. Decreased expression of HLA class II antigens on human uveal melanoma cells after in vivo X-ray irradiation. Am J Ophthalmol. 1988 Jan 15;105(1):78–86. doi: 10.1016/0002-9394(88)90125-0. [DOI] [PubMed] [Google Scholar]
- Kaufman D. S., Schoon R. A., Robertson M. J., Leibson P. J. Inhibition of selective signaling events in natural killer cells recognizing major histocompatibility complex class I. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6484–6488. doi: 10.1073/pnas.92.14.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Testi R., Bindl J., Phillips J. H. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989 Jun 1;169(6):2233–2238. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Luyten G. P., Luider T. M., Niederkorn J. Y. Relationship between natural killer cell susceptibility and metastasis of human uveal melanoma cells in a murine model. Invest Ophthalmol Vis Sci. 1995 Feb;36(2):435–441. [PubMed] [Google Scholar]
- Natali P. G., Bigotti A., Nicotra M. R., Nardi R. M., Delovu A., Segatto O., Ferrone S. Analysis of the antigenic profile of uveal melanoma lesions with anti-cutaneous melanoma-associated antigen and anti-HLA monoclonal antibodies. Cancer Res. 1989 Mar 1;49(5):1269–1274. [PubMed] [Google Scholar]
- Ruiter D. J., Mattijssen V., Broecker E. B., Ferrone S. MHC antigens in human melanomas. Semin Cancer Biol. 1991 Feb;2(1):35–45. [PubMed] [Google Scholar]
- Whelchel J. C., Farah S. E., McLean I. W., Burnier M. N. Immunohistochemistry of infiltrating lymphocytes in uveal malignant melanoma. Invest Ophthalmol Vis Sci. 1993 Jul;34(8):2603–2606. [PubMed] [Google Scholar]
- Whiteside T. L., Herberman R. B. The role of natural killer cells in immune surveillance of cancer. Curr Opin Immunol. 1995 Oct;7(5):704–710. doi: 10.1016/0952-7915(95)80080-8. [DOI] [PubMed] [Google Scholar]
- de Waard-Siebinga I., Hilders C. G., Hansen B. E., van Delft J. L., Jager M. J. HLA expression and tumor-infiltrating immune cells in uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 1996 Jan;234(1):34–42. doi: 10.1007/BF00186516. [DOI] [PubMed] [Google Scholar]
- de Waard-Siebinga I., Kool J., Jager M. J. HLA antigen expression on uveal melanoma cells in vivo and in vitro. Hum Immunol. 1995 Oct;44(2):111–117. doi: 10.1016/0198-8859(95)00083-g. [DOI] [PubMed] [Google Scholar]
- van Duinen S. G., Ruiter D. J., Broecker E. B., van der Velde E. A., Sorg C., Welvaart K., Ferrone S. Level of HLA antigens in locoregional metastases and clinical course of the disease in patients with melanoma. Cancer Res. 1988 Feb 15;48(4):1019–1025. [PubMed] [Google Scholar]


