Abstract
BACKGROUND/AIMS—Bacterial endotoxin is a potent inflammatory stimulator, the local and systemic responses thereby elicited being mediated via the release of cytokines from diverse cell types. Under physiological conditions, the corneal endothelium is protected from these toxins by the epithelial and vascular barriers, but in organ culture these safeguards are no longer operative, and such substances will therefore have ready access to this cell layer. The consequences of such exposure may take the form of overt damage to the endothelium and/or a more discreet influence on the cornea's immunological status, the effects of which may be realised only after transplantation, by its poor performance. The media bathing organ cultured donor corneas were monitored for the presence of various cytokine mediators of the inflammatory response before and after incubation with endotoxin, and these data compared with those pertaining to endothelial cell morphology and numerical density. METHODS—Six pairs of fellow donor corneas were cultured for an initial equilibration period of 10 days and then transferred to fresh medium; thereafter, one of each pair was incubated in the absence, and the other in the presence, of endotoxin (50 µg/ml ≡ 25 000 units/ml), and culturing continued for a further 10 days. Samples of medium were withdrawn at regular intervals throughout the 20 days and screened for the cytokines IL-1, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, GM-CSF, and TNF by ELISA; endothelial cell morphology and area density were assessed on days 0, 10, and 20. RESULTS—Spiking of organ culture media with endotoxin led to a substantial increase in the level of IL-8, and a smaller one in that of IL-6, but none of the other cytokines were detected. In five of the six stimulated corneas, these changes coincided with an increased incidence of endothelial cell loss, compared with that incurred by the fellow control, and the surviving population also evinced signs of degeneration not seen in the latter. CONCLUSION—Endotoxin induced increases in the levels of IL-6 and IL-8 appear to be correlated with endothelial cell loss. Since no adverse effects of this toxin on long term cultured monolayers of human corneal endothelial cells have been previously observed, the damage incurred in corneal organ culture may well be attributable to the influence of cytokines produced by other corneal cells or a non-intrinsic (passenger) cell population, such as macrophages, Langerhans cells, or lymphocytes present under these latter conditions.
Full Text
The Full Text of this article is available as a PDF (235.2 KB).
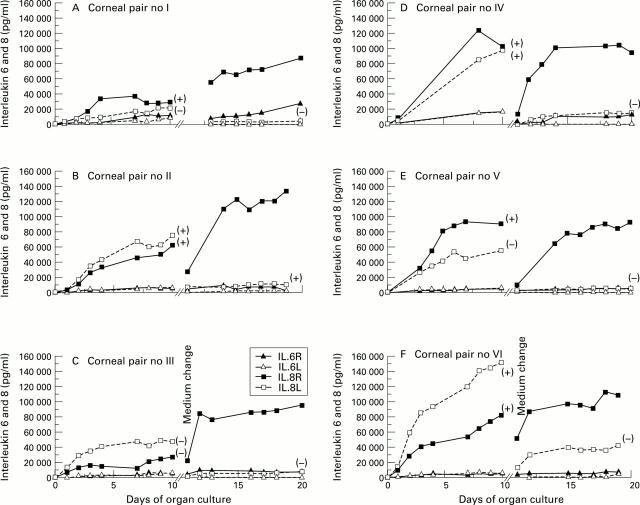
Figure 1 .
Levels of IL-6 (triangles) and IL-8 (squares) accumulating in the media of cultured corneas, data pertaining to each of the six fellow pairs (I to VI) being represented in (A) to (F), respectively. Traces through solid symbols correspond to right corneal media; those through open symbols to left ones. After an initial 10 day equilibrium phase, the medium bathing each cornea was replaced (break in x axis), and those containing right corneas spiked with 25 000 units/ml of E coli endotoxin; left corneas served as controls. Symbols in parentheses indicate whether media registered negative (−) or positive (+) for donor derived endotoxin (see Table 1).
Figure 2 .
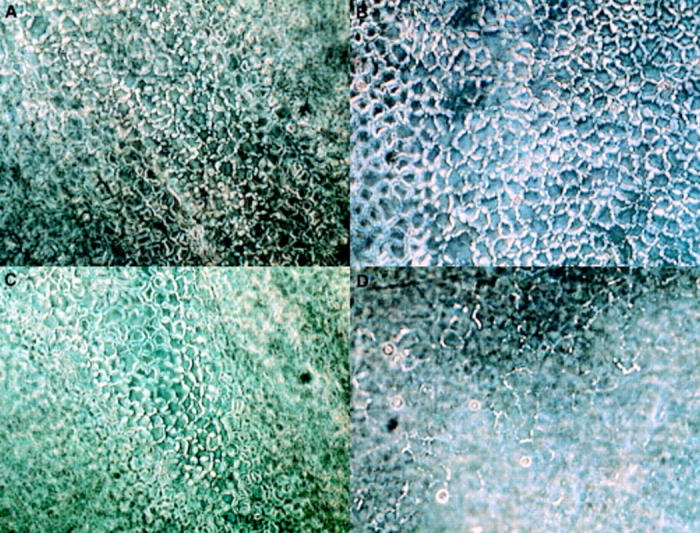
Appearance of left (A, B) and right (C, D) corneal endothelial cells (case no V), as seen in the phase contrast microscope, after an initial 10 day equilibration phase (A, C) and upon termination of the subsequent experimental one of equal duration (B, D), the onset of which was distinguished by change of medium and spiking of the right corneal culture with 25 000 units/ml of E coli endotoxin (D); the left corneal culture served as control (B). Left corneal endothelial cells exhibited a normal appearance over the entire 20 day culture period, as did right ones during the initial 10 day phase; but right corneal endothelial cells exposed to 25 000 units/ml of endotoxin manifested an increased incidence of polymorphism, cell enlargement, and incomplete intercellular border swelling (after immersion in buffered saline).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhnke M. Spendergewebe für die Keratoplastik. Erfahrungsbericht aus der Hamburger Hornhautbank. Klin Monbl Augenheilkd. 1991 Jun;198(6):562–571. doi: 10.1055/s-2008-1046033. [DOI] [PubMed] [Google Scholar]
- Doran J. E. Biological effects of endotoxin. Curr Stud Hematol Blood Transfus. 1992;(59):66–99. doi: 10.1159/000429609. [DOI] [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Pavilack M. A., Elner S. G., Remick D. G., Danforth J. M., Kunkel S. L. Human corneal interleukin-8. IL-1 and TNF-induced gene expression and secretion. Am J Pathol. 1991 Nov;139(5):977–988. [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Lowry S. F. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin Immunol Immunopathol. 1990 May;55(2):157–170. doi: 10.1016/0090-1229(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Frueh B. E., Böhnke M. Corneal grafting of donor tissue preserved for longer than 4 weeks in organ-culture medium. Cornea. 1995 Sep;14(5):463–466. [PubMed] [Google Scholar]
- Grabner G., Luger T. A., Smolin G., Oppenheim J. J. Corneal epithelial cell--derived thymocyte-activating factor (CETAF). Invest Ophthalmol Vis Sci. 1982 Dec;23(6):757–763. [PubMed] [Google Scholar]
- Hagenah M., Böhnke M., Engelmann K., Winter R. Incidence of bacterial and fungal contamination of donor corneas preserved by organ culture. Cornea. 1995 Jul;14(4):423–426. doi: 10.1097/00003226-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Hoekzema R., Verhagen C., van Haren M., Kijlstra A. Endotoxin-induced uveitis in the rat. The significance of intraocular interleukin-6. Invest Ophthalmol Vis Sci. 1992 Mar;33(3):532–539. [PubMed] [Google Scholar]
- Issekutz A. C., Megyeri P., Issekutz T. B. Role for macrophage products in endotoxin-induced polymorphonuclear leukocyte accumulation during inflammation. Lab Invest. 1987 Jan;56(1):49–59. [PubMed] [Google Scholar]
- Murray P. I., Hoekzema R., van Haren M. A., de Hon F. D., Kijlstra A. Aqueous humor interleukin-6 levels in uveitis. Invest Ophthalmol Vis Sci. 1990 May;31(5):917–920. [PubMed] [Google Scholar]
- Rosenbaum J. T., Wong K., Perez H. D., Raymond W., Howes E. L., Jr Characterization of endotoxin-induced C5-derived chemotactic activity in aqueous humor. Invest Ophthalmol Vis Sci. 1984 Oct;25(10):1184–1191. [PubMed] [Google Scholar]
- Sakamoto S., Inada K. [Human corneal epithelial, stromal and endothelial cells produce interleukin-6]. Nippon Ganka Gakkai Zasshi. 1992 Jun;96(6):702–709. [PubMed] [Google Scholar]
- Ulevitch R. J., Tobias P. S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Moore R. N., Sipe J. D., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980 Apr;124(4):2004–2009. [PubMed] [Google Scholar]
- Wahl L. M., Olsen C. E., Sandberg A. L., Mergenhagen S. E. Prostaglandin regulation of macrophage collagenase production. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4955–4958. doi: 10.1073/pnas.74.11.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]