Abstract
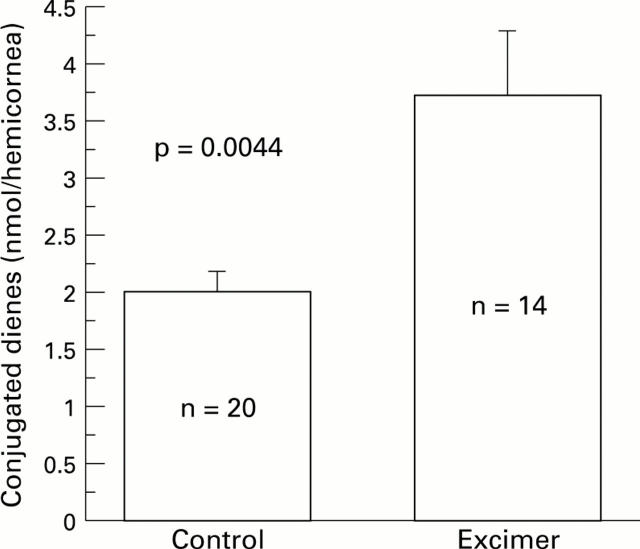
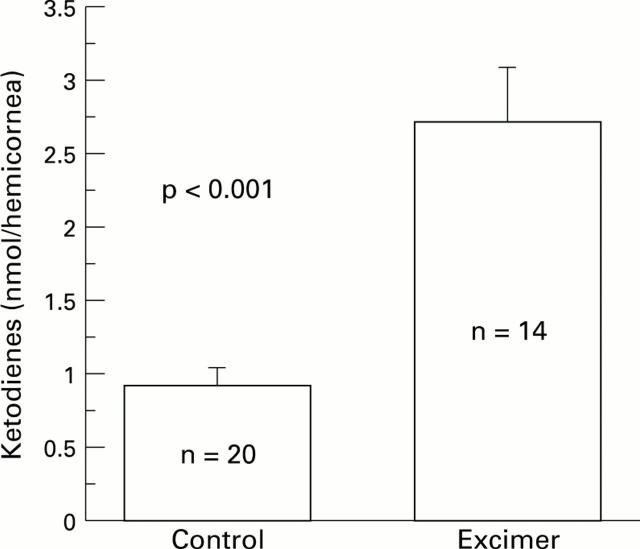
AIMS/BACKGROUND—To evaluate the extent of oxygen radical damage in the cornea after excimer laser ablation. METHODS—The 193 nm argon fluoride excimer laser was programmed for an average fluence of 150 mJ/cm2, with a firing rate of 5 Hz and an ablation zone diameter of 6 mm. Phototherapeutic keratectomy was performed to remove 30 µm of epithelium and 50 µm of stroma from the corneas of New Zealand white rabbits. Oxidative tissue damage after laser was determined by measuring oxidised lipids (conjugated dienes and ketodienes) in corneal lipid extracts, and by fast blue B staining to localise the lipid peroxide in the tissue. RESULTS—Conjugated diene levels were 3.73 (SD 0.56) nmol per hemicornea in ablated corneas and 1.99 (0.33) nmol per hemicornea in normal corneas (p = 0.0044). Ketodiene levels were 2.72 (0.38) nmol per hemicornea in treated corneas and 0.91 (0.12) nmol per hemicornea in normal corneas (p < 0.001). Fast blue B staining disclosed that the tissue damage occurred primarily on the surface of the ablated cornea. CONCLUSION—The presence of lipid peroxidation in the superficial corneal stroma in excimer laser treated corneas was demonstrated. This lipid peroxidation could be from oxygen free radicals generated by the infiltrating polymorphonuclear cells at the site of tissue damage.
Full Text
The Full Text of this article is available as a PDF (111.1 KB).
Figure 1 .
Rabbit corneas were subjected to excimer laser keratectomy with an average fluence of 150 mJ/cm2, a firing rate of 5 Hz and an ablation zone diameter of 6 mm. Conjugated dienes were measured in lipid extracts of the rabbit corneas 24 hours after excimer laser keratectomy. Control corneas were not treated.
Figure 2 .
Rabbit corneas were subjected to excimer laser keratectomy with an average fluence of 150 mJ/cm2, a firing rate of 5 Hz and an ablation zone diameter of 6 mm. Conjugated ketodienes were measured in lipid extracts of the rabbit corneas 24 hours after excimer laser keratectomy. Control corneas were not treated.
Figure 3 .
Haematoxylin and eosin staining of an excimer laser ablated cornea removed immediately after the treatment.
Figure 4 .
Haematoxylin and eosin staining showing the polymorphonuclear cells infiltrating the surface of an excimer laser ablated rabbit cornea 24 hours after excimer laser keratectomy.
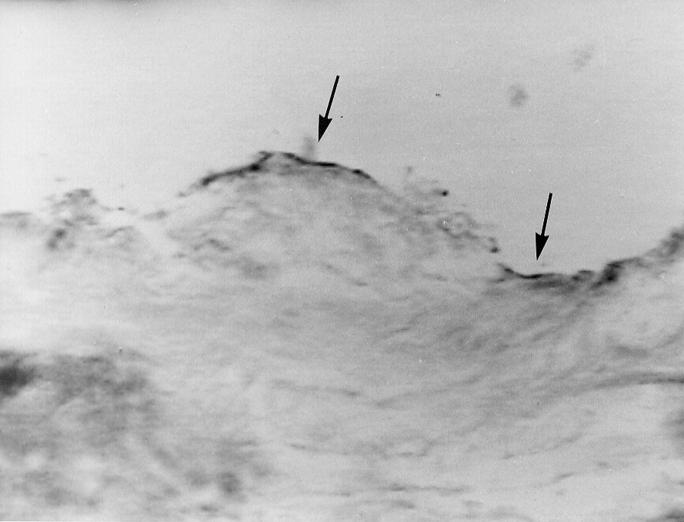
Figure 5 .
Photomicrograph of a corneal section where fast blue B staining shows lipid peroxide formation on the surface of the laser treated area (arrows) after 24 hours (original magnification × 100).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan H. E., Bazan N. G. Composition of phospholipids and free fatty acids and incorporation of labeled arachidonic acid in rabbit cornea. Comparison of epithelium, stroma and endothelium. Curr Eye Res. 1984 Nov;3(11):1313–1319. doi: 10.3109/02713688409007418. [DOI] [PubMed] [Google Scholar]
- Beuerman R. W., McDonald M. B., Shofner R. S., Munnerlyn C. R., Clapham T. N., Salmeron B., Kaufman H. E. Quantitative histological studies of primate corneas after excimer laser photorefractive keratectomy. Arch Ophthalmol. 1994 Aug;112(8):1103–1110. doi: 10.1001/archopht.1994.01090200109031. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Goto H., Wu G. S., Chen F., Kristeva M., Sevanian A., Rao N. A. Lipid peroxidation in experimental uveitis: sequential studies. Curr Eye Res. 1992 Jun;11(6):489–499. doi: 10.3109/02713689209001805. [DOI] [PubMed] [Google Scholar]
- Goto H., Wu G. S., Gritz D. C., Atalla L. R., Rao N. A. Chemotactic activity of the peroxidized retinal membrane lipids in experimental autoimmune uveitis. Curr Eye Res. 1991 Nov;10(11):1009–1014. doi: 10.3109/02713689109020339. [DOI] [PubMed] [Google Scholar]
- Lonn E., Factor S. M., Van Hoeven K. H., Wen W. H., Zhao M., Dawood F., Liu P. Effects of oxygen free radicals and scavengers on the cardiac extracellular collagen matrix during ischemia-reperfusion. Can J Cardiol. 1994 Mar;10(2):203–213. [PubMed] [Google Scholar]
- Malley D. S., Steinert R. F., Puliafito C. A., Dobi E. T. Immunofluorescence study of corneal wound healing after excimer laser anterior keratectomy in the monkey eye. Arch Ophthalmol. 1990 Sep;108(9):1316–1322. doi: 10.1001/archopht.1990.01070110132037. [DOI] [PubMed] [Google Scholar]
- McDonald M. B., Frantz J. M., Klyce S. D., Beuerman R. W., Varnell R., Munnerlyn C. R., Clapham T. N., Salmeron B., Kaufman H. E. Central photorefractive keratectomy for myopia. The blind eye study. Arch Ophthalmol. 1990 Jun;108(6):799–808. doi: 10.1001/archopht.1990.01070080041033. [DOI] [PubMed] [Google Scholar]
- McDonnell P. J., Moreira H., Clapham T. N., D'Arcy J., Munnerlyn C. R. Photorefractive keratectomy for astigmatism. Initial clinical results. Arch Ophthalmol. 1991 Oct;109(10):1370–1373. doi: 10.1001/archopht.1991.01080100050041. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay C. K., Chatterjee I. B. Free metal ion-independent oxidative damage of collagen. Protection by ascorbic acid. J Biol Chem. 1994 Dec 2;269(48):30200–30205. [PubMed] [Google Scholar]
- Phillips A. F., Szerenyi K., Campos M., Krueger R. R., McDonnell P. J. Arachidonic acid metabolites after excimer laser corneal surgery. Arch Ophthalmol. 1993 Sep;111(9):1273–1278. doi: 10.1001/archopht.1993.01090090125030. [DOI] [PubMed] [Google Scholar]
- Pompella A., Comporti M. The use of 3-hydroxy-2-naphthoic acid hydrazide and Fast Blue B for the histochemical detection of lipid peroxidation in animal tissues--a microphotometric study. Histochemistry. 1991;95(3):255–262. doi: 10.1007/BF00266775. [DOI] [PubMed] [Google Scholar]
- Rao N. A. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc. 1990;88:797–850. [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Bellavite P., Berton G., Grzeskowiak M., Papini E. Mechanism of production of toxic oxygen radicals by granulocytes and macrophages and their function in the inflammatory process. Pathol Res Pract. 1985 Aug;180(2):136–142. doi: 10.1016/S0344-0338(85)80161-8. [DOI] [PubMed] [Google Scholar]
- Sher N. A., Bowers R. A., Zabel R. W., Frantz J. M., Eiferman R. A., Brown D. C., Rowsey J. J., Parker P., Chen V., Lindstrom R. L. Clinical use of the 193-nm excimer laser in the treatment of corneal scars. Arch Ophthalmol. 1991 Apr;109(4):491–498. doi: 10.1001/archopht.1991.01080040059027. [DOI] [PubMed] [Google Scholar]
- Stark W. J., Chamon W., Kamp M. T., Enger C. L., Rencs E. V., Gottsh J. D. Clinical follow-up of 193-nm ArF excimer laser photokeratectomy. Ophthalmology. 1992 May;99(5):805–812. doi: 10.1016/s0161-6420(92)31896-2. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]