Abstract
AIM—Many growth factors are implicated in proliferative diabetic retinopathy (PDR). It was decided to test the hypothesis that no one factor is predominant but that a regular profile of levels of different growth factors might be operating, and that the profile might differ according to whether or not insulin therapy was part of the patient's glycaemic management. The levels of several growth factors in vitrectomy samples were therefore determined from diabetic patients with tractional, non-haemorrhagic sequelae of PDR and these levels were correlated with (a) each other (growth factor profile), (b) neovascular activity, and (c) the method of glycaemic management (insulin treated (IT) or non-insulin treated (NIT)). METHODS—72 samples of vitreous were obtained from either diabetic patients with PDR (n = 51) or non-diabetic (control) patients (n = 21). Levels of bFGF, IGF-I, EGF, and insulin were determined by radioimmunoassay; levels of TGF-β2 by ELISA; and levels of IGF-I binding protein by western ligand blotting. The data were analysed using appropriate statistics. RESULTS—There was no regular growth factor profile. bFGF levels were significantly greater in vitreous from NIT patients compared with IT patients and controls. The highest levels of bFGF were found in NIT patients with actively vascularised membranes. TGF-β2 levels were significantly greater in vitreous from IT patients compared with NIT patients and controls The highest levels of TGF-β2 were found in IT patients with actively vascularised membranes. IGF-I levels were significantly greater in diabetics (irrespective of insulin treatment) than non-diabetics and the highest levels of IGF-I were found in IT patients with actively vascularised membranes. A 34 kDa IGFBP was the predominant IGFBP identified in vitreous and was found to be elevated in diabetics patients. CONCLUSION—In PDR there is a correlation between intravitreal growth factor levels and both disease state (whether active or fibrotic) and method of glycaemic management.
Full Text
The Full Text of this article is available as a PDF (168.6 KB).
Figure 1 .
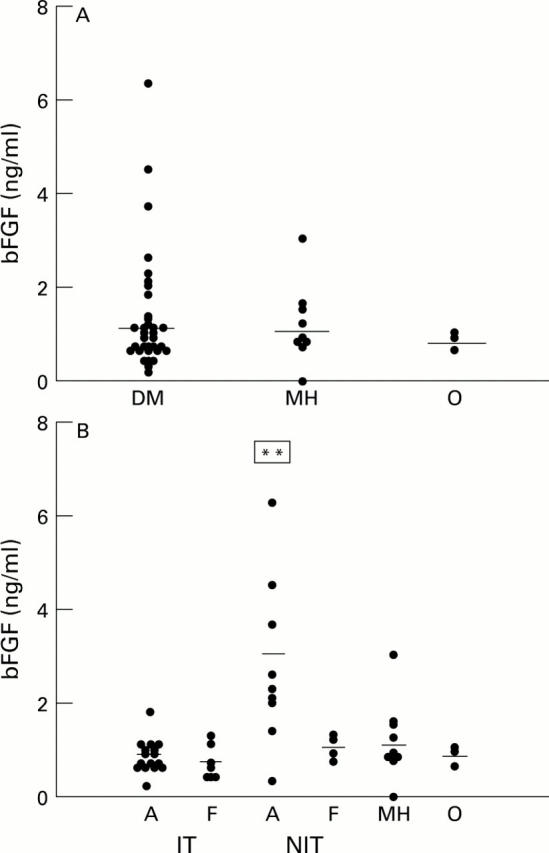
Scatter plots of bFGF levels in vitreous samples from diabetic (with proliferative diabetic retinopathy) and non-diabetic patients. (A) All diabetic samples (DM) versus non-diabetic samples (MH = macular hole, O = rhegmatogenous retinal detachment). (B) Diabetic samples subdivided according to glycaemic management and activity of neovascularisation (A = active, F = fibrotic). Horizontal bar = mean of each subgroup. **Significantly different (p<0.05) from the other groups.
Figure 2 .
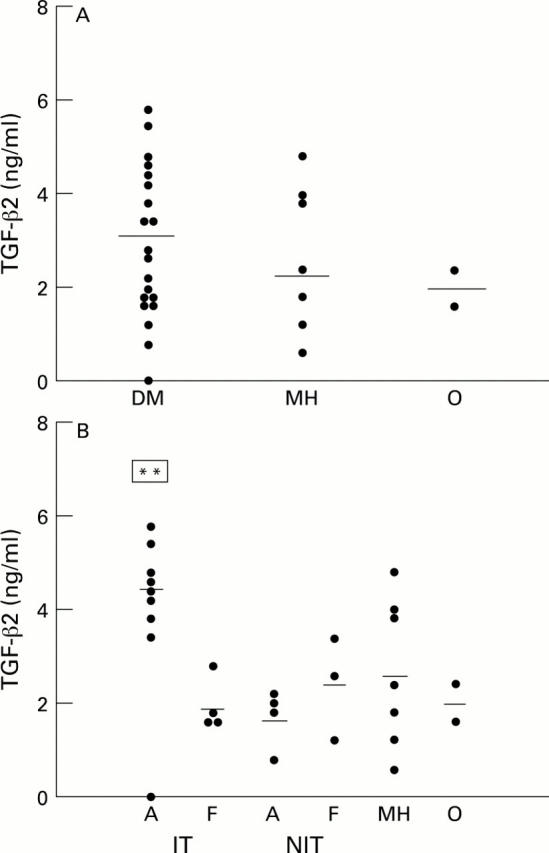
Scatter plots of TGF-β levels in vitreous samples from diabetic (with proliferative diabetic retinopathy) and non-diabetic patients. (A) All diabetic samples (DM) versus non-diabetic samples (MH = macular hole, O = rhegmatogenous retinal detachment). (B) Diabetic samples subdivided according to glycaemic management and activity of neovascularisation (A = active, F = fibrotic). Horizontal bar = mean of each subgroup. **Significantly different (p<0.05) from the other subgroups.
Figure 3 .
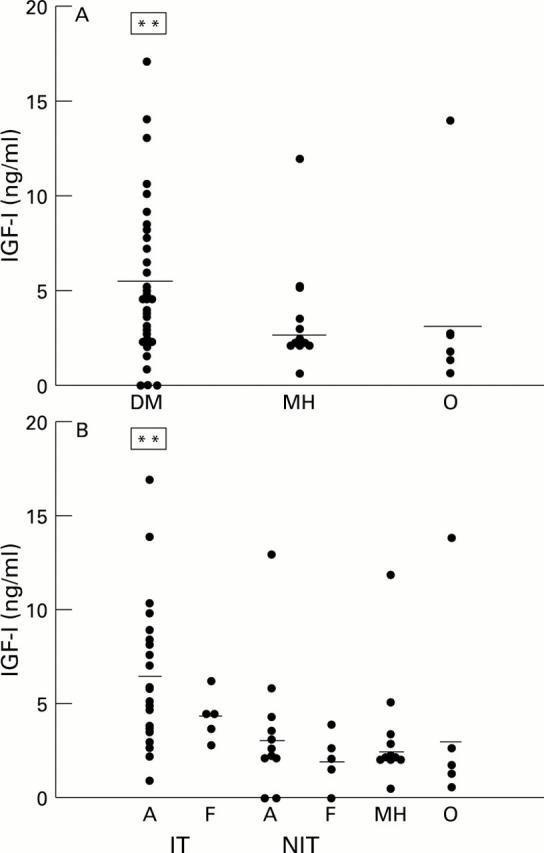
Scatter plots of IGF-I levels in vitreous samples from diabetic (with proliferative diabetic retinopathy) and non-diabetic patients. (A) All diabetic samples (DM) versus non-diabetic samples (MH = macular hole, O = rhegmatogenous retinal detachment). (B) Diabetic samples subdivided according to glycaemic management and activity of neovascularisation (A = active, F = fibrotic). Horizontal bar = mean of each subgroup. **Significantly different (p<0.05) from the other subgroups.
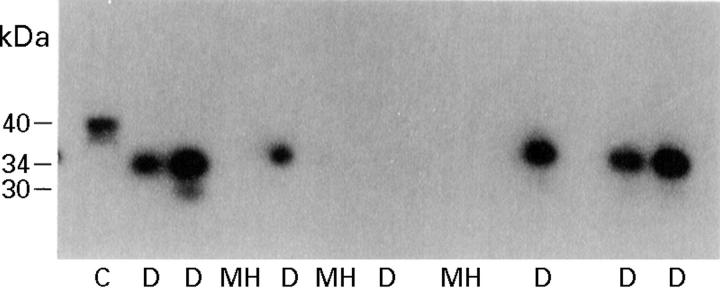
Figure 4 .
Western ligand blot of insulin-like growth factor binding proteins present in vitreous samples from patients with diabetic retinopathy (D) and non-diabetic patients with macular hole (MH). The data presented are representative of all the samples analysed. C= human plasma run as a control. The estimated Mr (kDa) of each form was derived by comparison with defined Mr standards.
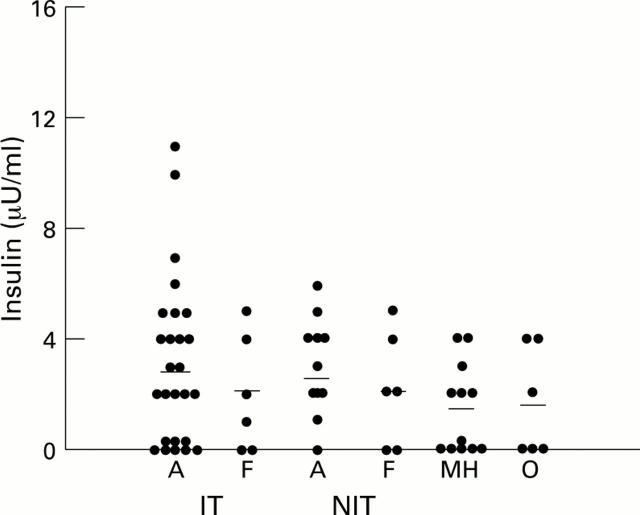
Figure 5 .
Scatter plots of insulin levels in vitreous samples from diabetic (with proliferative diabetic retinopathy) and non-diabetic patients. Diabetic samples subdivided according to glycaemic management and activity of neovascularisation (A = active neovascularisation, F = fibrotic). MH = macular hole, O = rhegmatogenous retinal detachment. Horizontal bar = mean of each group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu el Asrar A. M., Maimone D., Morse P. H., Gregory S., Reder A. T. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 1992 Dec 15;114(6):731–736. doi: 10.1016/s0002-9394(14)74052-8. [DOI] [PubMed] [Google Scholar]
- Adamis A. P., Miller J. W., Bernal M. T., D'Amico D. J., Folkman J., Yeo T. K., Yeo K. T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994 Oct 15;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Aiello L. P., Avery R. L., Arrigg P. G., Keyt B. A., Jampel H. D., Shah S. T., Pasquale L. R., Thieme H., Iwamoto M. A., Park J. E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec 1;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Arnold D. R., Moshayedi P., Schoen T. J., Jones B. E., Chader G. J., Waldbillig R. J. Distribution of IGF-I and -II, IGF binding proteins (IGFBPs) and IGFBP mRNA in ocular fluids and tissues: potential sites of synthesis of IGFBPs in aqueous and vitreous. Exp Eye Res. 1993 May;56(5):555–565. doi: 10.1006/exer.1993.1069. [DOI] [PubMed] [Google Scholar]
- Baird A., Culler F., Jones K. L., Guillemin R. Angiogenic factor in human ocular fluid. Lancet. 1985 Sep 7;2(8454):563–563. doi: 10.1016/s0140-6736(85)90512-4. [DOI] [PubMed] [Google Scholar]
- Battegay E. J. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med (Berl) 1995 Jul;73(7):333–346. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- Boulton M. E., McLeod D., Garner A. Vasoproliferative retinopathies: clinical, morphogenetic and modulatory aspects. Eye (Lond) 1988;2 (Suppl):S124–S139. doi: 10.1038/eye.1988.139. [DOI] [PubMed] [Google Scholar]
- Boulton M., Moriarty P., Gregor Z. J. Biological activities of vitreous gel, retrohyaloid fluid and subretinal fluid from diabetic and non-diabetic eyes. Br J Ophthalmol. 1992 Feb;76(2):79–83. doi: 10.1136/bjo.76.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M., Patel B., Khaliq A., Moriarty P., Jarvis-Evans J., McLeod D. Modulators and milieu in preretinal neovascularisation. Eye (Lond) 1992;6(Pt 6):560–565. doi: 10.1038/eye.1992.122. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R. IGF binding proteins and their functions. Mol Reprod Dev. 1993 Aug;35(4):368–375. doi: 10.1002/mrd.1080350409. [DOI] [PubMed] [Google Scholar]
- Connor T. B., Jr, Roberts A. B., Sporn M. B., Danielpour D., Dart L. L., Michels R. G., de Bustros S., Enger C., Kato H., Lansing M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989 May;83(5):1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danias J., Stylianopoulou F. Expression of IGF-I and IGF-II genes in the adult rat eye. Curr Eye Res. 1990 Apr;9(4):379–386. doi: 10.3109/02713689008999626. [DOI] [PubMed] [Google Scholar]
- Hanneken A., Baird A. Soluble forms of the high-affinity fibroblast growth factor receptor in human vitreous fluid. Invest Ophthalmol Vis Sci. 1995 May;36(6):1192–1196. [PubMed] [Google Scholar]
- Khaliq A., Patel B., Jarvis-Evans J., Moriarty P., McLeod D., Boulton M. Oxygen modulates production of bFGF and TGF-beta by retinal cells in vitro. Exp Eye Res. 1995 Apr;60(4):415–423. doi: 10.1016/s0014-4835(05)80098-3. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., D'Amore P. A. Regulators of angiogenesis. Annu Rev Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- Malecaze F., Clamens S., Simorre-Pinatel V., Mathis A., Chollet P., Favard C., Bayard F., Plouet J. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994 Nov;112(11):1476–1482. doi: 10.1001/archopht.1994.01090230090028. [DOI] [PubMed] [Google Scholar]
- Meyer-Schwickerath R., Pfeiffer A., Blum W. F., Freyberger H., Klein M., Lösche C., Röllmann R., Schatz H. Vitreous levels of the insulin-like growth factors I and II, and the insulin-like growth factor binding proteins 2 and 3, increase in neovascular eye disease. Studies in nondiabetic and diabetic subjects. J Clin Invest. 1993 Dec;92(6):2620–2625. doi: 10.1172/JCI116877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty P., Boulton M., Dickson A., McLeod D. Production of IGF-I and IGF binding proteins by retinal cells in vitro. Br J Ophthalmol. 1994 Aug;78(8):638–642. doi: 10.1136/bjo.78.8.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B., Hiscott P., Charteris D., Mather J., McLeod D., Boulton M. Retinal and preretinal localisation of epidermal growth factor, transforming growth factor alpha, and their receptor in proliferative diabetic retinopathy. Br J Ophthalmol. 1994 Sep;78(9):714–718. doi: 10.1136/bjo.78.9.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G. S., Grant M. B. Neovascular growth factors. Eye (Lond) 1991;5(Pt 2):170–180. doi: 10.1038/eye.1991.31. [DOI] [PubMed] [Google Scholar]
- Sharp P. S. The role of growth factors in the development of diabetic retinopathy. Metabolism. 1995 Oct;44(10 Suppl 4):72–75. doi: 10.1016/0026-0495(95)90224-4. [DOI] [PubMed] [Google Scholar]
- Singh A., Boulton M., Lane C., Forrester J., Gaal J., McLeod D. Inhibition of microvascular endothelial cell proliferation by vitreous following retinal scatter photocoagulation. Br J Ophthalmol. 1990 Jun;74(6):328–332. doi: 10.1136/bjo.74.6.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivalingam A., Kenney J., Brown G. C., Benson W. E., Donoso L. Basic fibroblast growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1990 Jun;108(6):869–872. doi: 10.1001/archopht.1990.01070080113046. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Tang S., Scheiffarth O. F., Thurau S. R., Wildner G. Cells of the immune system and their cytokines in epiretinal membranes and in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmic Res. 1993;25(3):177–185. doi: 10.1159/000267287. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Kissun R. D., Schor A. M., McLeod D., Garner A., Weiss J. B. Endothelial cell-stimulating angiogenesis factor in vitreous from extraretinal neovascularizations. Invest Ophthalmol Vis Sci. 1989 Oct;30(10):2174–2178. [PubMed] [Google Scholar]
- Waldbillig R. J., Jones B. E., Schoen T. J., Moshayedi P., Heidersbach S., Bitar M. S., van Kuijk F. J., de Juan E., Kador P., Chader G. J. Vitreal insulin-like growth factor binding proteins (IGFBPs) are increased in human and animal diabetics. Curr Eye Res. 1994 Jul;13(7):539–546. doi: 10.3109/02713689408999886. [DOI] [PubMed] [Google Scholar]
- de Boer J. H., Limpens J., Orengo-Nania S., de Jong P. T., La Heij E., Kijlstra A. Low mature TGF-beta 2 levels in aqueous humor during uveitis. Invest Ophthalmol Vis Sci. 1994 Sep;35(10):3702–3710. [PubMed] [Google Scholar]