Abstract
AIM—The effect of breathing 100% oxygen on retinal and optic nerve head capillary blood flow in smokers and non-smokers was investigated using scanning laser Doppler flowmetry (SLDF) as a new non-invasive method to visualise and quantify ocular blood flow. METHOD—10 eyes of 10 young healthy non-smoking volunteers (mean age 26 (SD 3) years) and nine eyes of nine young healthy smoking volunteers (mean age 26 (4) years) were investigated. All participants were asked not to smoke or consume caffeine containing drinks for at least 4 hours before the measurements. Blood flow measurements were performed before and after 100% oxygen was applied to the subjects through a mask over a period of 5 minutes (6 litres per minute). Juxtapapillary retinal and optic nerve head blood flow were determined in arbitrary units using SLDF representing a combination of laser Doppler flowmetry and a scanning laser system allowing visualisation and quantification of the retinal and optic nerve head blood flow. Blood flow was determined in an area of 100 µm × 100 µm. The level of carboxyhaemoglobin was determined in all subjects. A Wilcoxon matched pairs signed ranks test (non-parametric) was used for statistical evaluation. RESULTS—In the non-smoking group, retinal `flow' was reduced by 33% (p = 0.005), optic nerve head `flow' by 37% (p = 0.005). In the smoking group retinal flow was reduced by 10% (p = 0.01), optic nerve head flow by 13% (p <0.008). The difference in reactivity to oxygen breathing between smokers and non-smokers was highly significant (p <0.00001). Increased carboxyhaemoglobin levels were not found in either of the groups. A significant reduction of the mean arterial blood pressure of 6% (5%) (p <0.02) was observed in the non-smoking group after administration of oxygen. CONCLUSION—These results indicate that hyperoxia leads to a decrease in capillary blood flow of the retina and optic nerve head secondary to vasoconstriction, and that smokers do not respond to oxygen breathing as non-smokers do. The findings might be based on factors such as long term effects of nicotine on the sympathetic and parasympathetic nervous system.
Full Text
The Full Text of this article is available as a PDF (186.7 KB).
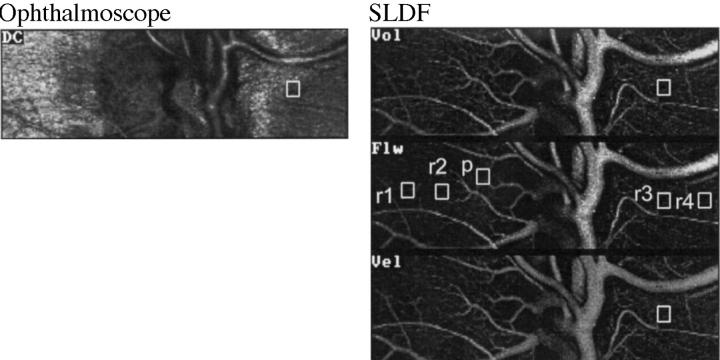
Figure 1 .
Detail magnification. Above: ophthalmoscopic image (DC); right: images of volume (Vol), flow (Flw), and velocity (Vel). The brightness of pixels in these images is in accordance with the values of volume, flow, and velocity, respectively. Right centre: flow image: square, variable in size (here 10 × 10 pixels), and possible locations for quantitative evaluation (r1-r4 = retina, p = optic nerve head); once the square is positioned at a certain location this location will be the same in all images (as demonstrated in r3 and in Fig 2) leading to values of volume, flow, and velocity of one location.
Figure 2 .
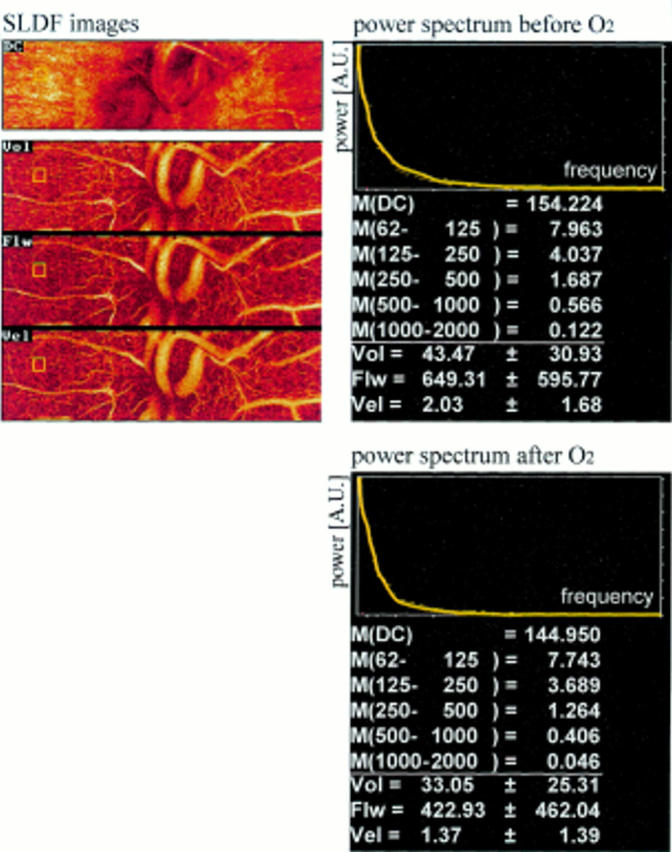
Scanning laser Doppler flowmetry images with power spectra before and after breathing oxygen. Above (from top to bottom): ophthalmoscopic image images volume (Vol), flow (Flw), velocity (Vel). Right, top: power spectra. Bottom: M(DC) = mean power of DC value (the DC value is proportional to the power of the reflected non-frequency shifted light). M(62-125) = mean power in the frequency ranges 62 Hz to 125 Hz lines below mean power in respective frequency ranges.
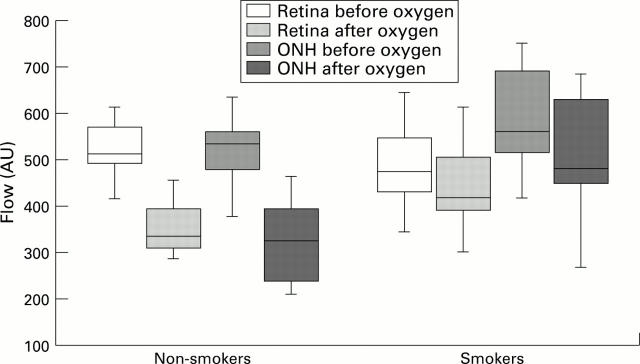
Figure 3 .
Graph of reactivity changes (flow) as boxplot. Box contains 50% of the values falling between the 25th and 75th percentiles; line in the box indicates the median. Vertical lines extend from the box to the highest and lowest values (excluding outliers and extreme values). This resembles approximately two standard deviations.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cryer P. E., Haymond M. W., Santiago J. V., Shah S. D. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976 Sep 9;295(11):573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- Deutsch T. A., Read J. S., Ernest J. T., Goldstick T. K. Effects of oxygen and carbon dioxide on the retinal vasculature in humans. Arch Ophthalmol. 1983 Aug;101(8):1278–1280. doi: 10.1001/archopht.1983.01040020280023. [DOI] [PubMed] [Google Scholar]
- FRAYSER R., HICKAM J. B. RETINAL VASCULAR RESPONSE TO BREATHING INCREASED CARBON DIOXIDE AND OXYGEN CONCENTRATIONS. Invest Ophthalmol. 1964 Aug;3:427–431. [PubMed] [Google Scholar]
- Fallon T. J., Maxwell D., Kohner E. M. Retinal vascular autoregulation in conditions of hyperoxia and hypoxia using the blue field entoptic phenomenon. Ophthalmology. 1985 May;92(5):701–705. doi: 10.1016/s0161-6420(85)33978-7. [DOI] [PubMed] [Google Scholar]
- Klein B. E., Klein R., Ritter L. L. Relationship of drinking alcohol and smoking to prevalence of open-angle glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1993 Nov;100(11):1609–1613. doi: 10.1016/s0161-6420(93)31429-6. [DOI] [PubMed] [Google Scholar]
- Marshall G., Garg S. K., Jackson W. E., Holmes D. L., Chase H. P. Factors influencing the onset and progression of diabetic retinopathy in subjects with insulin-dependent diabetes mellitus. Ophthalmology. 1993 Aug;100(8):1133–1139. doi: 10.1016/s0161-6420(13)31517-6. [DOI] [PubMed] [Google Scholar]
- Michelson G., Groh M., Langhans M., Schmauss B. Zweidimensionale Kartierung der retinalen und papillären Mikrozirkulation mittels Scanning-Laser-Doppler-Flowmetrie. Klin Monbl Augenheilkd. 1995 Sep;207(3):180–190. doi: 10.1055/s-2008-1035365. [DOI] [PubMed] [Google Scholar]
- Michelson G., Schmauss B., Langhans M. J., Harazny J., Groh M. J. Principle, validity, and reliability of scanning laser Doppler flowmetry. J Glaucoma. 1996 Apr;5(2):99–105. [PubMed] [Google Scholar]
- Michelson G., Schmauss B. Two dimensional mapping of the perfusion of the retina and optic nerve head. Br J Ophthalmol. 1995 Dec;79(12):1126–1132. doi: 10.1136/bjo.79.12.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado P. B., Chen H. C., Patel V., Herbert L., Kohner E. M. The acute effect of smoking on retinal blood flow in subjects with and without diabetes. Ophthalmology. 1994 Jul;101(7):1220–1226. doi: 10.1016/s0161-6420(94)31185-7. [DOI] [PubMed] [Google Scholar]
- Moss S. E., Klein R., Klein B. E. Association of cigarette smoking with diabetic retinopathy. Diabetes Care. 1991 Feb;14(2):119–126. doi: 10.2337/diacare.14.2.119. [DOI] [PubMed] [Google Scholar]
- Riva C. E., Grunwald J. E., Sinclair S. H. Laser Doppler Velocimetry study of the effect of pure oxygen breathing on retinal blood flow. Invest Ophthalmol Vis Sci. 1983 Jan;24(1):47–51. [PubMed] [Google Scholar]
- Robinson F., Petrig B. L., Riva C. E. The acute effect of cigarette smoking on macular capillary blood flow in humans. Invest Ophthalmol Vis Sci. 1985 May;26(5):609–613. [PubMed] [Google Scholar]