Abstract
Selection of particular antigen-derived peptides by class II MHC molecules determines the population of complexes represented on the antigen-presenting cell surface and available for T cell receptor engagement. This discriminating selection process results from unique interactions between the spectrum of peptides generated during antigen processing and the MHC molecules. Here, we examined the selection of peptides by the class II MHC, I-Ak. Our results indicate that although peptide primary anchors are key in MHC binding, auxiliary anchors are a powerful regulatory component in the selection of peptides by I-Ak. Study of the segments surrounding the dominant hen egg white lysozome(48-61) epitope demonstrates that auxiliary anchors also are involved in determining the binding register of I-Ak along an extended peptide. In addition, we found that unique combinations of auxiliary anchors can act in concert to modulate the binding of peptides to I-Ak.
The cellular and biochemical basis of class II MHC peptide selection, specifically of the I-Ak molecule, is a major concern of our laboratory. From a large pool of available proteins, MHC molecules select hundreds of different peptides to present on the cell surface. However, each individual MHC allele has its own specificity and binds a select subset of the peptides generated during antigen processing (1–4). For instance, antigen-presenting cells (APC) cultured with hen egg white lysozyme (HEL) predominantly present only four HEL epitopes on I-Ak (reviewed in refs. 5 and 6). The aim of our present investigation is to determine the biochemical basis for the selection of chemically dominant epitope families.
Several characteristics of the peptides bound to I-Ak inform us of key features involved in epitope selection. First, selected peptides are presented as a nested family with a core segment containing variable-length extensions (7–12). This core segment usually is represented by a nine residue peptide that makes two sets of interactions with the MHC molecule: one via peptide backbone hydrogen bonds to conserved MHC residues, the other by peptide side-chain-specific interactions with polymorphic pocket-lining residues (reviewed in ref. 13). With some MHC molecules, one or two anchor side chains may dominate the binding interaction, and are therefore considered primary anchors. The remaining peptide anchors make a minimal contribution to binding; these are referred to as auxiliary anchors. Concerning the flanking residues, variability of their lengths increases the diversity of the peptide family. Flanking residues can also contribute to MHC binding affinity and provide additional T cell contacts (14–17). Second, the selective presentation of MHC/peptide complexes is not equivalent, but encompasses a range from peptides that are abundant, to those represented by only a few molecules (9, 12, 18, 19). Characterization of numerous autologous peptides presented by class II indicates that approximately 40% are present in relatively high concentrations (20), usually consisting of more than 1,000 molecules per APC. The remaining peptides are found with few copies per APC.
With respect to HEL, there is one major dominant epitope family that can occupy up to 20% of the APC's I-Ak molecules, HEL(48-61) (10, 21). An extensive analysis of this I-Ak immunodominant epitope identified Asp52 as its primary anchor, the single position most involved in I-Ak high affinity binding, SDS resistance, and persistence on APC (22, 23). Indeed, the I-Ak/HEL(48-61) crystal structure demonstrated that this Asp52 residue fits precisely within the I-Ak P1 pocket, whereas the other anchor side chains were loosely accommodated in the remaining pockets (24). Having an Asp at P1, the latter anchors in this peptide contribute a minor degree to binding, and are therefore considered auxiliary anchors.
Concerning HEL(48-61), because of Asp52's tight interaction with the P1 pocket and its role in binding, we speculated that the presence of Asp, as well as Asn, primary anchors served as a rationale for the abundant selection of major I-Ak epitopes. Likewise, there is a preponderance of Asp and Asn residues at the fourth or fifth position of numerous naturally processed epitopes (10, 25). According to this motif, proteins like HEL would possess multiple putative epitopes corresponding to each of these residues.
Our laboratory biochemically identified that I-Ak predominantly selects four HEL epitopes: (18-33), (34-47), (48-62), and (115-129) (ref. 26, and reviewed in ref. 6). It was found, by limiting dilution analysis of HEL-immunized mice, that T cells specific for these four epitopes account for 80–90% of the total HEL-reactive cells. At this point, we do not know whether each segment is recognized in more than one register, an issue crucial to revealing the entire repertoire of T cell epitopes of an antigen. Moreover, we question whether nonprimary anchor residues influence I-Ak selection and binding of peptides bearing putative P1 anchors.
Materials and Methods
Peptide Synthesis and Radiolabeling.
Peptides were synthesized by using fluorenylmethoxycarbonyl chemistry on a Symphony peptide synthesizer (Rainen Instruments), purified to homogeneity by reverse phase HPLC, and analyzed by matrix-assisted laser desorption ionization–time of flight. Some peptides were labeled on internal Tyr residues with NaI125 by the chloramine-T method, as described by Nelson et al. (27).
Relative Binding Affinity Measurements.
PBS (2.5 μl) containing 50 mM l-lysophosphatidylcholine and 20 mM MEGA-8 and MEGA-9, 7.5 μl of 1 M 2-N-morpholino ethane sulfonic acid at pH 5.5, 0.125 pmol of iodonated reference peptide (sequence YEDYGILQINSR), and 12.5 pmol of detergent-solubilized I-Ak (purified according to ref. 28) were mixed in 1.5-ml tubes. Test peptide (5 μl) was added to the reactions in increasing concentrations. After a 48-h room temperature incubation, the MHC/peptide complexes were separated from unbound peptide with Bio-Spin 6 chromatography columns (Bio-Rad) and individually evaluated for bound 125I-labeled peptide with a γ counter. The amount of test peptide necessary to inhibit 50% of the reference peptide's binding was determined. These values were normalized to the concentration of unlabeled reference peptide required to complete 50% of the labeled reference peptide's binding [relative inhibitory concentration (RIC)−1 = concentrationtest/concentrationstandard]. A small RIC−1 value indicates strong relative binding, whereas a large RIC−1 value indicates weak relative binding.
Aw Antibody Immunoprecipitation of I-Ak/Peptide Complexes.
Substitutions of Tyr53 with Phe and the addition of a C-terminal Tyr to HEL(48-61) peptides were made to preserve Aw3.18 binding to iodinated peptides (21). These 125I-labeled peptides were bound to purified I-Ak as described above, but without competitor peptide. Each immunoprecipitation contained roughly 50,000 cpm of Bio-spin-purified I-Ak/peptide complexes in 100 μl of PBS with 1% Triton X-100. Various doses of the Aw3.18 antibody (up to 75 μg) were added to the mixtures and incubated for 2 h at 4°C. Subsequently, 50 μl of a 50% slurry of protein G-Sepharose in PBS with 1% Trition X-100 was added to each precipitation reaction. The mixtures were incubated for an additional hour at 4°C. Antibody-bound complexes were separated from nonreactive complexes by centrifugation through oil columns consisting of 60% (vol/vol) dibutyl phthalate and 40% (vol/vol) dioctyl phthalate.
SDS/PAGE Stability of I-Ak/Peptide Complexes.
The ability of individual peptides to stabilize I-Ak heterodimers migrating through SDS/PAGE gels was determined by using 125I-labeled peptide bound to purified I-Ak, as described above. The complexes were immunoprecipitated with 25 μg/ml of 40 F anti-I-Akβ monoclonal antibody for 2 h at 4°C (23), captured with protein A-Sepharose beads, and then incubated with 100 μl of SDS/sample buffer for 1 h at room temperature. Lastly, the I-Ak/peptide complexes were run on SDS/10% PAGE gels. Gels were dried and analyzed by phosphorimaging. Labeled peptides migrated either with the αβ heterodimer (stable) or dissociated from it and ran at the bottom of the gel (unstable).
I-Ak/Peptide Complex Off-Rate.
I-Ak complexes were formed with 125I-labeled peptides and subsequently purified with P6 Bio-spin columns. An excess (5 nmol) of unlabeled peptide (sequence YEDYGILQINSR) was added to inhibit rebinding of the radiolabeled peptides. Aliquots of the purified complexes were subjected to additional separations with P6 Bio-spin columns at specified time points and evaluated by γ counting. The remaining cpm of bound peptide at each time point was expressed as a percentage of the initial amount of bound peptide (t = 0).
3A9 T Cell Hybridoma Assays.
The ability of the 3A9 I-Ak/HEL(48-61)-restricted T cell to recognize I-Ak complexes formed with variant HEL peptides was studied: 5 × 104 live M12.C3.F6 APC and 1 × 105 3A9 T cell hybridoma cells were mixed with different doses of test peptide in 200-μl microtiter wells for 18 h at 37°C with 5% CO2. Aliquots (100 μl) of each well were transferred to new microtiter plates, frozen, and thawed. 2 × 104 CTLL, IL-2 indicator cells, in a 100-μl volume were added to each well and incubated for an additional 18 h. After that, 2 μCi (1 Ci = 37 GBq) of [3H]thymidine were added to each well and incubated for 7 h. Finally, the cells were harvested on paper filters with a 96-well harvester (Skatron, Sterling, VA) and analyzed for radioactivity by scintillation β counting.
Results
I-Ak Binds the HEL(42-61) Segment Although a Single Register.
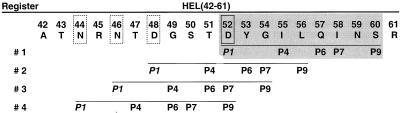
The major family of selected HEL peptides usually starts at residue Asp48 and ends at Trp62 or Trp63. We asked whether HEL(48-62) contains two binding registers, having either the Asp52, described previously, or the Asp48 as P1 anchors. We also questioned the apparent lack of naturally selected peptides extending beyond Asn46 or Asn44, which may serve as potential P1 anchors. Accordingly, there may be four potential binding registers within an extended HEL(42-61) segment (Fig. 1), each possessing candidate primary anchors for I-Ak P1 pocket binding: Asn44, Asn46, Asp48, and Asp52 (corresponding to register 1). With respect to T cell recognition, binding through these different putative registers would generate distinct T cell epitopes from a single peptide segment.
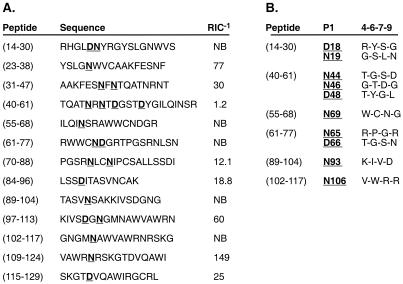
Figure 1.
Four potential I-Ak binding registers of HEL(42-61). On the unfolding of HEL during antigen processing, the I-Ak P1 pocket has the potential to bind to one of four Asp or Asn residues within HEL(42-61), resulting in four separate registers. These four registers are underlined with the predicted P1 anchors in italics and corresponding auxiliary pockets labeled P4, P6, P7, and P9. Register 1 of the immunodominant HEL(48-61) peptide (shaded box) is established by Asp52 (solid-outline box) anchoring at the P1 pocket. The three additional putative P1 anchors, Asn44, Asn46, and Asp48 are shown in dotted-outline boxes.
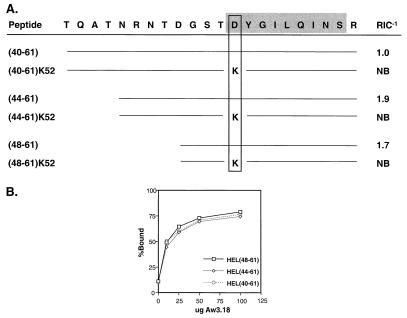
We examined the binding of each of these four overlapping registers by two methods. First, the relative affinity of different N-terminal length peptides was compared with that of HEL(48-61) to determine the effect of multiple peptide registers on I-Ak binding (Fig. 2A). All possessed similar relative affinities for I-Ak. This finding led us to substitute the Asp52 P1 anchor with Lys, an inhibitory residue that prohibits register 1 binding (20). Blocking register 1 binding with a Lys52 substitution completely inhibited the binding of all of the HEL(48-61)-containing peptides.
Figure 2.
I-Ak exclusively binds extended HEL(48-61) peptides through one single register. (A) A Lys52 P1 anchor inhibited the binding of HEL(48-61), blocking register 1. Prevention of register 1 binding with Lys52 substitutions inhibited the binding of the extended HEL(48-61) peptides containing three additional putative P1 anchors. (B) The register 1-specific antibody, Aw3.18, was used to immunoprecipitate I-Ak complexes individually formed with radiolabeled HEL(40-61), HEL(44-61), or HEL(48-61) peptides. Despite the presence of four potential binding registers, the Aw3.18 antibody equally immunoprecipitated I-Ak complexes with the extended HEL peptides. Consequently, I-Ak bound the extended HEL(40-61) and HEL(44-61) peptides through the same single register as HEL(48-61).
Second, we selectively immunoprecipitated purified I-Ak complexes formed with iodinated peptides of different N-terminal lengths, HEL(48-61), HEL(44-61), or HEL(40-61), by using the Aw3.18 monoclonal antibody (Fig. 2B). The Aw3.18 antibody reacts only with I-Ak complexes bound to HEL(48-61)-containing peptides in which the P1 pocket harbors the Asp52 anchor, i.e., register 1 (21). We expected that the binding of the extended peptides to I-Ak through multiple registers, for example registers 2, 3, or 4, would decrease the number of Aw3.18-reactive complexes. Nonetheless, an equivalent amount of material was immunoprecipitated with each reaction. We concluded from these two approaches that HEL(40-61), HEL(44-61), and HEL(48-61) form complexes only through register 1.
Specific Combinations of Peptide Auxiliary Anchors Prevent I-Ak Binding.
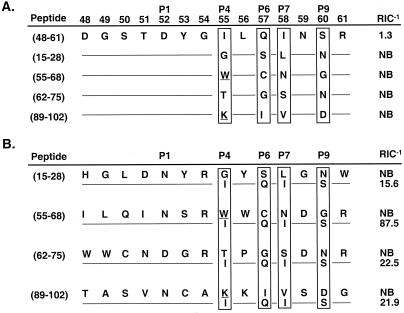
We then considered that the auxiliary anchors corresponding to each of the alternative registers may reduce peptide binding. First, we explored the specificity of I-Ak pockets by examining the binding of individual HEL(48-61) variants with single P4, P6, P7, or P9 auxiliary anchor substitutions (Fig. 3). Peptides were made in which each HEL(48-61) auxiliary anchor was substituted separately with different residues. Most substitutions did not change or had only subtle effects on HEL(48-61) binding to I-Ak. However, the P4 pocket did not accept Lys, Trp, or Tyr, whereas the P6 pocket was sensitive to Lys, implying that I-Ak selects against peptides containing these residues at the P4 and P6 anchor positions. Likewise, Lys was not favored at the P7 or P9 anchor positions.
Figure 3.
I-Ak auxiliary pockets tolerate a wide range of peptide anchors. HEL(48-61) auxiliary anchors were individually substituted with aliphatic, nonpolar, polar, and charged residues. The relative binding affinities of these peptides for I-Ak is listed on the right. Highlighted are the individual HEL(48-61) auxiliary anchor substitutions that alone were able to completely prevent peptide binding despite the presence of the strong P1 Asp52: P4 substitutions with Lys, Trp, and Tyr, and P6 substitution with Lys. NB, nonbinding.
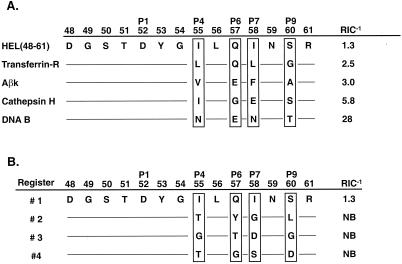
Second, we substituted all four auxiliary anchors of HEL(48-61) (P4 Ile, P6 Gln, P7 Ile, and P9 Ser) with the corresponding auxiliary anchors from four peptides known to bind to I-Ak to determine the ability of favorable anchors to be exchanged. These auxiliary anchors were derived from the transferrin receptor (10), DNA B (10), cathepsin H (25), and Aβk molecule (25) naturally processed epitopes (Fig. 4A). For instance, the Aβk peptide P4 Val, P6 Glu, P7 Phe, and P9 Ala anchors replaced the analogous HEL(48-61) auxiliary anchors, resulting in the chimeric peptide: DGSTDYVLEFNAR. The auxiliary anchors from the different epitopes were exchangeable, for the chimeric HEL(48-61) peptides bound I-Ak with equal or improved relative affinities as their naturally processed counterparts.
Figure 4.
Favorable and unfavorable auxiliary anchors affect peptide binding. (A) Favorable auxiliary anchors can be exchanged. The HEL(48-61) auxiliary anchors were replaced with those from four different natural I-Ak epitopes [transferrin receptor () KGTDFQLNQLEGKKGY (ref. 10), DNA B () DPFKGDDFNEENPTEPS (ref. 10), Aβk (37–53) YVRFDSDVGEERAVTEL (ref. 25), and cathepsin H (77–92) YILYNKGIMGEDSYPY (ref. 25)], all of which contained a similar strong P1 anchor, either Asp or Asn (23). The resulting hybrid peptides bound I-Ak with similar or improved RIC−1 values as the original natural peptides: transferrin-R RIC−1 = 14, Aβk = 2, cathepsin H = 23, and DNA B = 28. (B) HEL(48-61) auxiliary anchors were substituted with all four predicted anchors from each alternative register, 2, 3, or 4 (Fig. 1) and were tested for I-Ak binding. Replacement of HEL(48-61) auxiliary anchors (register 1) with those of registers 2, 3, or 4 completely blocked peptide binding, even though individual substitutions with each anchor had little effect on peptide binding.
Third, knowing that auxiliary anchors from binding epitopes could be exchanged and preserve I-Ak binding, we analyzed the binding of peptides in which the HEL(48-61) auxiliary anchors were substituted together with the four corresponding auxiliary anchors derived from each putative register 2, 3, and 4 of Fig. 1 (Fig. 4B). The complete replacement of the four HEL(48-61) auxiliary anchors with those from any of the three other registers prevented binding.
Auxiliary Anchor Substitutions Modify I-Ak/HEL(48-61) Complexes.
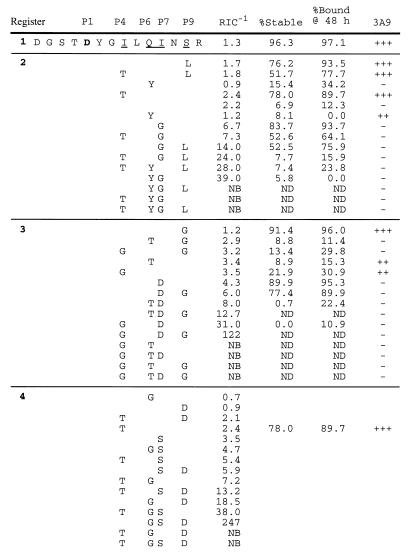
Fig. 5 demonstrates the effects of single or combined HEL(48-61) auxiliary anchor substitutions with the corresponding P4, P6, P7, and P9 anchors of registers 2, 3, or 4. For substitutions with the auxiliary anchors of registers 2 and 3, we examined several binding parameters: relative affinity, off-rate, and resistance to SDS denaturation. [For I-Ak, the denaturation of the complex by SDS/PAGE is a reliable parameter of peptide binding strength. Weak interactions result in complexes with a higher propensity to dissociate, whereas the contrary holds for many strong-binding peptides (6, 16, 29)].
Figure 5.
Hindering auxiliary anchors modifies I-Ak binding and recognition. Register 1 HEL(48-61) P4, P6, P7, and P9 anchors were substituted with one, two, three, or four auxiliary anchors from each of the alternative registers (2, 3, or 4) and analyzed for binding. HEL(48-61) substitutions with register 2 and 3 auxiliary anchors also were analyzed for SDS/PAGE stability and half-life. Additionally, register 2- and 3-substituted HEL(48-61) peptides were incubated with I-Ak-bearing APC and offered to 3A9 T cell hybridomas. +++, maximal IL-2 response; ++, moderate IL-2 response; −, no IL-2 response. The maximal response corresponded to approximately 40,000–60,000 cpm at 0.1 μM, whereas moderate required 10- to 50-fold more peptide for a similar response. NB, nonbinding; ND, not determined because of poor binding.
Individually, no single anchor substitution inhibited binding, but several altered the interaction with the complex. For example, a P4 Thr from register 2 or 4 placed at the hydrophobic P4 pocket had a minor negative effect on binding and dissociation rate, accompanied by a slight decrease in the SDS-resistance of the complex compared with the wild-type peptide. This result is in contrast to a Gly P4 substitution from register 3, which had a moderate effect on the relative binding affinity, but significantly increased both the off-rate and the sensitivity of the complex to SDS denaturation. In a similar manner, P6 substitution with the corresponding P6 anchor from either register 2 or 3, Tyr and Thr respectively, slightly affected the relative binding but negatively altered the SDS-resistance and the off-rate of the complexes. In the case of the I-Ak P7 pocket, the predicted P7 anchors from each of the three alternative registers had subtle negative effects on peptide relative affinity. The P7 Gly and Asp of registers 2 and 3 had only moderate negative effects on complex persistence and SDS-resistance. P9 anchor substitutions from the alternative registers were the most tolerated and did not modify significantly the binding properties of the complex.
Although separate substitutions had only a mild effect, combinations of two, three, or four substitutions compounded the reduction in binding. Note the effects from the register 2 Tyr at P6 together with Gly at P7 (Fig. 5). An additional substitution of either P4 with Thr or P9 with Leu completely abrogated binding. It is noteworthy that binding was most sensitive to multiple substitutions, which included Gly at P4, P6, or P7.
We also determined the responsiveness of the 3A9 T cell hybridoma to HEL(48-61) substitutions with register 2 and 3 auxiliary anchors. According to the I-Ak crystal structure (24), these anchor side-chain substitutions should not be directly available for contacting the T cell antigen receptor, but instead be buried within I-Ak pockets. The T cell hybridoma was virtually unresponsive to substitutions with register 2 or 3 auxiliary anchors, and recognized only single P4, P6, and P9 substitutions. Therefore, the 3A9 T cell hybridoma had an exquisite sensitivity to alterations of the HEL(48-61) complex induced by auxiliary anchor substitutions. The ability of auxiliary anchor substitutions to modify T cell responses has been reported previously, including in refs. 30 and 31.
I-Ak Selectively Binds Putative HEL Epitopes.
Realizing that auxiliary anchors could inhibit the binding of overlapping peptide registers in the HEL(42-61) segment, we investigated their overall role in the selection of HEL epitopes by I-Ak. Based on the HEL sequence, we created a panel of synthetic peptides in which the fourth or fifth residue from the N terminus corresponded to an Asp or an Asn, the two P1 anchor residues found in high frequency among the naturally selected I-Ak peptides (Fig. 6A; refs. 6, 10, and 25). Several of these potential epitopes contained multiple Asp or Asn candidate primary anchors. In these cases, separate Ala-substituted peptides at each predicted primary anchor position were examined. These peptides were tested for binding and found to have a wide spectrum of relative binding affinities. Of the 21 potential Asp or Asn-bearing epitopes only 4 bound well to I-Ak. HEL(48-61) bound I-Ak with the highest relative affinity, whereas the remaining three had binding strengths 10- to 25-fold less.
Figure 6.
I-Ak selectively binds Asp- and Asn-bearing HEL epitopes. (A) I-Ak binds a limited subset of the putative HEL epitopes. There are 21 Asp and Asn residues within HEL that may act as possible P1 anchors for I-Ak binding. Synthetic HEL peptides of these potential epitopes were tested for I-Ak binding. Of these putative epitopes, 11 had no detectable binding, 6 bound weakly, and 4 bound well to I-Ak. (B) Many Asp- and Asn-containing HEL peptides are unable to bind I-Ak. Shown are the P1 and auxiliary anchors corresponding to these nonbinding (NB) peptides. For example, HEL(97-110) possesses a suitable P1 Asp101, with G-N-A-V auxiliary anchors, but was unable to bind I-Ak. Peptides with overlapping potential epitopes were tested with individual Ala substitutions and are grouped together.
The specific auxiliary anchor combinations of the nonbinding peptides potentially explains their lack of selection by I-Ak during processing and the lack of T cells specific to them. Several of the nonbinding peptides possessed either a basic or large aromatic at P4 or basic residue at P6, which prevented peptide binding (Fig. 6B). For example, Asp18 as the P1 of HEL(14-30) would produce the P4, P6, P7, and P9 anchor combination Arg-Tyr-Ser-Gly. The P4 Arg likely blocked the binding of this peptide. However, the same HEL(14-30) peptide could alternatively enter the P1 pocket with Asn19, generating the auxiliary anchor combination Gly-Ser-Leu-Asn. Replacing HEL(48-61) Ile-Gln-Ile-Ser auxiliary anchors with those of HEL(14-30), Gly-Ser-Leu-Asn, ablated the high-affinity binding of the HEL(48-61) peptide (Fig. 7A). The binding of similar chimeric HEL(48-61) peptides with their auxiliary anchors replaced with those of HEL(55-68), HEL(62-75), and HEL(89-102) also was inhibited. Trp62 of HEL(55-68) and Lys96 of HEL(89-102) were themselves likely to be inhibitory, whereas the combination of Gly-Ser-Leu-Asn of HEL(55-68) and Thr-Gly-Ser-Asn of HEL(62-75) resemble the negative residues described from HEL(42-61) registers 2 and 4 (Fig. 5).
Figure 7.
Hindering auxiliary anchors participate in I-Ak epitope selection. (A) Auxiliary anchors of several nonbinding potential HEL epitopes are responsible for inhibiting I-Ak binding. Substitution of the auxiliary anchors from four predicted HEL epitopes into the high-affinity HEL(48-61) peptide completely prohibited binding to I-Ak. HEL(55-68), and HEL(89-102) contained a single inhibitory residue (underlined), Trp and Lys, respectively, that alone is likely to block binding (as shown in Fig. 3). However, HEL(15-28) and HEL(62-75) did not contain any of these individual inhibitory residues, therefore, their combinations of auxiliary anchors were hindering. (B) Replacing the inhibitory residues of potential HEL epitopes with favorable auxiliary anchors imparted the ability to bind I-Ak. The auxiliary anchors of four nonbinding peptides were replaced with favorable anchors derived from HEL(48-61). These chimeric peptides gained the ability to bind I-Ak. However, these peptides did not bind with the same affinity as the natural HEL(48-61) peptide did, likely because of additional effects of nonanchor residues.
In the reverse situation, HEL(14-30)'s Gly-Ser-Leu-Asn auxiliary anchor combination was replaced with HEL(48-61)'s favorable anchors Ile-Gln-Ile-Ser (Fig. 7B). These anchors enabled the binding of HEL(14-30). A similar manipulation was performed with the HEL(55-68), HEL(62-75), and HEL(89-102) peptides. The favorable Ile-Gln-Ile-Ser also enabled the binding of these normally nonbinding peptides, albeit at a lower binding strength.
Discussion
We have demonstrated that the presence of a strong primary anchor interaction alone was not sufficient for peptide binding and that there was a significant role for auxiliary anchors in epitope selection. Auxiliary anchors, singly or together, did not add to binding strength. Indeed, a polyalanine peptide bearing an Asp P1 anchor bound as well as the wild-type HEL(48-61) peptide (23). However, our analysis highlighted that auxiliary anchors, individually or collectively in specific combinations, contributed to the binding of peptides with strong primary anchors. Furthermore, the interplay of auxiliary pockets collectively fine tuned the specificity of epitope selection and restricted the number of possible I-Ak binding registers along a peptide.
Here we see that the most significant auxiliary anchor affects on binding, stability, and peptide half-life were centered at the P4 and P6 pockets, which form at the interface of the I-Ak α and β chains. The stability of I-Ak may have a predisposed susceptibility to unfavorable pocket and anchor interactions at this site. Likewise, I-Ak stability also is exceptionally sensitive to P1 pocket interactions (23).
Our results indicated that basic P4 anchors were strongly inhibitory to peptide binding. This negative effect may have arisen from the inability of the hydrophobic and medium-sized P4 pocket to accommodate the charge and size of basic side chains. Tyr and Trp P4 anchors also blocked peptide binding, possibly by their bulky sizes. The smaller size of acidic residues may explain why negatively charged anchors were tolerated more readily at this pocket. In comparison, a small Gly P4 anchor only slightly decreased I-Ak relative affinity. However, a Gly P4 anchor severely reduced both the stability and half-life of the complex. This reduction may have been a result of several missing hydrophobic interactions formed by other hydrophobic anchors, for instance Ile or Ala (24, 32). Moreover, Gly anchors may have left an empty P4 pocket cavity to be filled by water, which likely is less favorable than occupying it with a larger, hydrophobic side chain.
The P6 pocket is formed by both hydrophobic and hydrophilic residues that impart a preference for polar and acidic anchors. Basic P6 anchors completely inhibited peptide binding, potentially by interfering with the Hisβ9 positioned at the base of the pocket. The I-Ag7 P6 pocket, which also bears a Hisβ9 (33), disfavors basic residues as well (unpublished data). Although a P6 Thr did not disrupt peptide binding significantly, as a Thr anchor did at the P6 pocket of DR1 (34), it did decrease the half-life and SDS resistance of I-Ak complexes substantially. A P6 Thr likely lacked the two hydrogen bonds formed by Gln, shown in the I-Ak/HEL(48-62) structure (24), potentially weakening the interaction with the pocket. Its hydroxyl also may have conflicted with that of Thrα65, lining the top of the pocket. In a similar manner, a Tyr anchor at the P6 pocket decreased complex stability, but not at the cost of relative affinity. A large Tyr P6 anchor could have raised the peptide backbone within the binding groove, to be accommodated at the P6 pocket. This backbone repositioning potentially could have compromised hydrogen bonds between the P6 amine and the Asnα62 and/or P7 amine with Tyrβ30, both of which are frequently observed among class II molecules (13). Another possible scenario may be that the Tyr avoided being buried within the P6 pocket and was more solvent-exposed. Similarly, the I-Ad structures demonstrated that peptide binding could occur even when the P1 and P9 anchors were not buried completely within their corresponding pockets (35). Nevertheless, contorting the peptide conformation to avoid nonfavorable anchor interactions with particular pockets may not have been conducive to the proper positioning of the remaining anchors.
The P7 and P9 pockets are more distant from the α and β chain interface than are the P4 and P6 pockets (24). Accordingly, their anchor substitutions had fewer effects on binding. Nonetheless, the P7 pocket, lined with predominantly hydrophobic residues, was mildly to moderately sensitive to Gly, acidic, basic, and polar anchors. A Gly anchor at the P7 pocket would have lacked several hydrophobic side-chain interactions with the pocket formed by the Ile and Trp anchors of the I-Ak structures. It also may encourage unfavorably the packing of water along the hydrophobic ledge, or the rearrangement of the pocket's residues to exclude water. Arg and Lys anchors would have been poised to have a possibly negative interaction with Argβ70 lining the top of the pocket. However, the I-Ak crystal structures indicated that Argβ70 was solvent-exposed, therefore, it may have adopted a conformation that may not necessarily have conflicted with an Arg or Lys peptide anchor (24, 32). In the case of the P9 pocket, Lys slightly decreased peptide relative affinity, potentially by interfering with Argα76.
I-Ak tolerated single unfavorable anchors. However, when these anchors were found together within a peptide, they synergized to disrupt peptide binding, even in the presence of a strong primary anchor. Therefore, we consider these auxiliary anchors “hindering” because the severity of their negative effects on binding was manifested when they were found together in specific combinations. Although other studies have reported on single negative anchor residues (31, 36–38), the striking effect noted here was the negative cooperativity among them, especially those combinations that included a Gly. A similar interdependence of different anchors has been demonstrated in the H2-Kb class I system in which the specificity of the B and C pocket was coupled (39).
We have demonstrated that I-Ak auxiliary pockets had the ability to accommodate a diversity of anchors, potentially broadening the spectrum of peptides that could bind. However, these auxiliary pockets also used an intricate mechanism of anchor discrimination that resulted in the selective presentation of peptides. In conclusion, we found that I-Ak auxiliary pockets refine the specificity of peptide selection, providing them with a powerful role in epitope selection.
Acknowledgments
We thank the National Institutes of Health for their continuous support of our work, including the graduate work of R.R.L. We appreciate Dr. Paul Allen's and Dr. Daved Fremont's insightful conversations. We also thank The Lucille P. Markey Program in Pathobiology for its support of R.R.L.
Abbreviations
- APC
antigen-presenting cell
- HEL
hen egg white lysozyme
- RIC−1
relative inhibitory concentration
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210384197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210384197
References
- 1.Babbitt B P, Allen P M, Matsueda G, Haber E, Unanue E R. Nature (London) 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- 2.Barcinski M A, Rosenthal A S. J Exp Med. 1977;145:726–742. doi: 10.1084/jem.145.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buus S, Sette A, Colon S M, Miles C, Grey H M. Science. 1987;235:1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- 4.Katz M E, Maizels R M, Wicker L, Miller A, Sercarz E E. Eur J Immunol. 1982;12:535–540. doi: 10.1002/eji.1830120702. [DOI] [PubMed] [Google Scholar]
- 5.Allen P M, Babbitt B P, Unanue E R. Immunol Rev. 1987;98:171–187. doi: 10.1111/j.1600-065x.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 6.Latek R R, Unanue E R. Immunol Rev. 1999;172:209–228. doi: 10.1111/j.1600-065x.1999.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 7.Chicz R M, Urban R G, Lane W S, Gorga J C, Stern L J, Vignali D A, Strominger J L. Nature (London) 1992;358:764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 8.Hunt D F, Michel H, Dickinson T A, Shabanowitz J, Cox A L, Sakaguchi K, Appella E, Grey H M, Sette A. Science. 1992;256:1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- 9.Gugasyan R, Vidavsky I, Nelson C A, Gross M L, Unanue E R. J Immunol. 1998;161:6074–6083. [PubMed] [Google Scholar]
- 10.Nelson C A, Roof R W, McCourt D W, Unanue E R. Proc Natl Acad Sci USA. 1992;89:7380–7383. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudensky A, Preston-Hurlburt P, Hong S C, Barlow A, Janeway C A., Jr Nature (London) 1991;353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 12.Vignali D A, Urban R G, Chicz R M, Strominger J L. Eur J Immunol. 1993;23:1602–1607. doi: 10.1002/eji.1830230731. [DOI] [PubMed] [Google Scholar]
- 13.Nelson C A, Fremont D H. Rev Immunogenet. 1999;1:47–59. [PubMed] [Google Scholar]
- 14.Carson R T, Vignali K M, Woodland D L, Vignali D A. Immunity. 1997;7:387–399. doi: 10.1016/s1074-7613(00)80360-x. [DOI] [PubMed] [Google Scholar]
- 15.Malcherek G, Gnau V, Stevanovic S, Rammensee H G, Jung G, Melms A. J Immunol. 1994;153:1141–1149. [PubMed] [Google Scholar]
- 16.Nelson C A, Petzold S J, Unanue E R. Proc Natl Acad Sci USA. 1993;90:1227–1231. doi: 10.1073/pnas.90.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vignali D A, Strominger J L. J Exp Med. 1994;179:1945–1956. doi: 10.1084/jem.179.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demotz S, Grey H M, Appella E, Sette A. Nature (London) 1989;342:682–684. doi: 10.1038/342682a0. [DOI] [PubMed] [Google Scholar]
- 19.Robadey C, Wallny H J, Demotz S. Eur J Immunol. 1996;26:1656–1659. doi: 10.1002/eji.1830260738. [DOI] [PubMed] [Google Scholar]
- 20.Gugasyan, R., Velazquez, C., Vidavsky, I., Deck, B. M., van der Drift, K., Gross, M. L. & Unanue, E. R. (2000) J. Immunol. 165, in press. [DOI] [PubMed]
- 21.Dadaglio G, Nelson C A, Deck M B, Petzold S J, Unanue E R. Immunity. 1997;6:727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 22.Nelson C A, Petzold S J, Unanue E R. Nature (London) 1994;371:250–252. doi: 10.1038/371250a0. [DOI] [PubMed] [Google Scholar]
- 23.Nelson C A, Viner N J, Young S P, Petzold S J, Unanue E R. J Immunol. 1996;157:755–762. [PubMed] [Google Scholar]
- 24.Fremont D H, Monnaie D, Nelson C A, Hendrickson W A, Unanue E R. Immunity. 1998;8:305–317. doi: 10.1016/s1074-7613(00)80536-1. [DOI] [PubMed] [Google Scholar]
- 25.Marrack P, Ignatowicz L, Kappler J W, Boymel J, Freed J H. J Exp Med. 1993;178:2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson D A, DiPaolo R J, Kanagawa O, Unanue E R. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 27.Nelson C A, Viner N, Young S, Petzold S, Benoist C, Mathis D, Unanue E R. J Immunol. 1996;156:176–182. [PubMed] [Google Scholar]
- 28.Luescher I F, Unanue E R. J Immunol Methods. 1990;135:233–245. doi: 10.1016/0022-1759(90)90277-3. [DOI] [PubMed] [Google Scholar]
- 29.Carrasco-Marin E, Petzold S, Unanue E R. J Biol Chem. 1999;274:31333–31340. doi: 10.1074/jbc.274.44.31333. [DOI] [PubMed] [Google Scholar]
- 30.Kersh G J, Kersh E N, Fremont D H, Allen P M. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 31.Boehncke W H, Takeshita T, Pendleton C D, Houghten R A, Sadegh-Nasseri S, Racioppi L, Berzofsky J A, Germain R N. J Immunol. 1993;150:331–341. [PubMed] [Google Scholar]
- 32.Reinherz E L, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey R E, Smolyar A, Hare B, Zhang R, et al. Science. 1999;286:1913–1921. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- 33.Latek R R, Suri A, Petzold S J, Nelson C A, Kanagawa O, Unanue E R, Fremont D H. Immunity. 2000;12:699–710. doi: 10.1016/s1074-7613(00)80220-4. [DOI] [PubMed] [Google Scholar]
- 34.Murthy V L, Stern L J. Structure (London) 1997;5:1385–1396. doi: 10.1016/s0969-2126(97)00288-8. [DOI] [PubMed] [Google Scholar]
- 35.Scott C A, Peterson P A, Teyton L, Wilson I A. Immunity. 1998;8:319–329. doi: 10.1016/s1074-7613(00)80537-3. [DOI] [PubMed] [Google Scholar]
- 36.Hammer J, Belunis C, Bolin D, Papadopoulos J, Walsky R, Higelin J, Danho W, Sinigaglia F, Nagy Z A. Proc Natl Acad Sci USA. 1994;91:4456–4460. doi: 10.1073/pnas.91.10.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert L E, Unanue E R. J Immunol. 1989;143:802–807. [PubMed] [Google Scholar]
- 38.Sette A, Sidney J, Oseroff C, del Guercio M F, Southwood S, Arrhenius T, Powell M F, Colon S M, Gaeta F C, Grey H M. J Immunol. 1993;151:3163–3170. [PubMed] [Google Scholar]
- 39.Fremont D H, Matsumura M, Stura E A, Peterson P A, Wilson I A. Science. 1992;257:919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]