Abstract
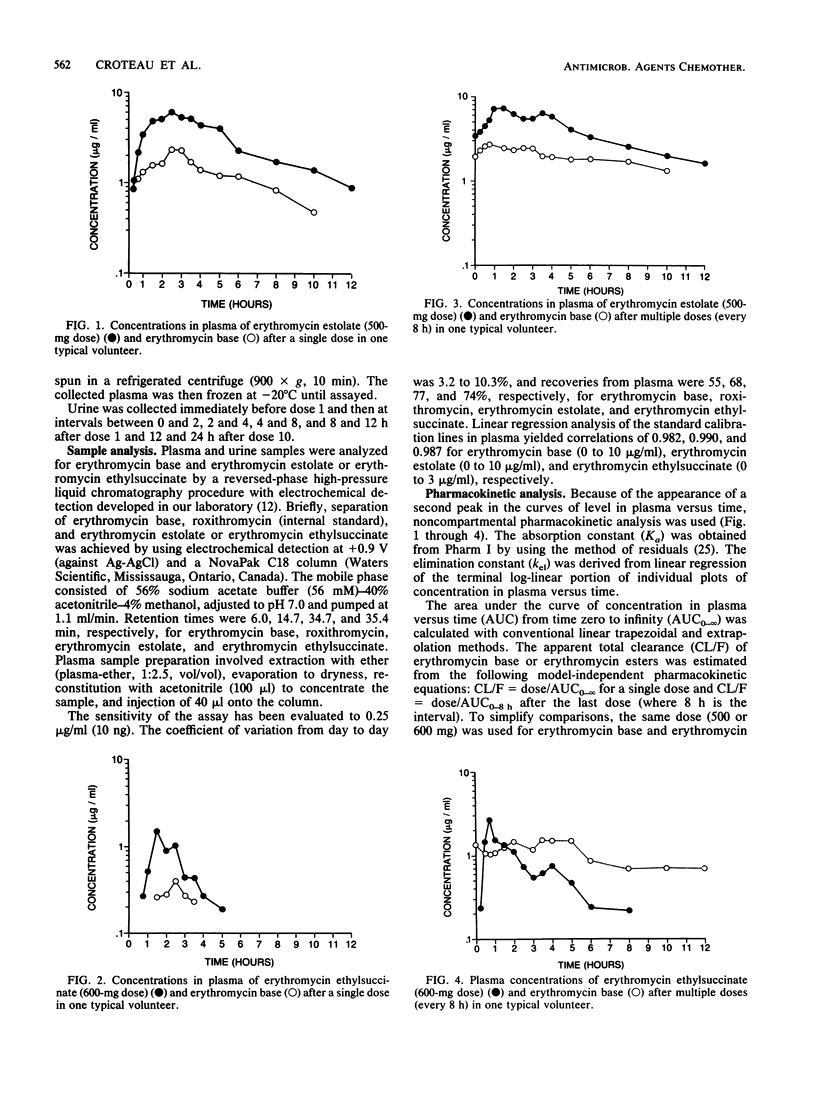
The pharmacokinetics of erythromycin estolate (500 mg) and erythromycin ethylsuccinate (600 mg) were compared in 12 healthy volunteers after single doses and after repeated oral doses (every 8 h). High-pressure liquid chromatography with electrochemical detection was used to determine concentrations in plasma and urine of estolate, ethylsuccinate, and erythromycin base. The maximum concentration of drug in the serum, the half-life, and the area under the curve for erythromycin estolate were significantly greater than those of erythromycin ethylsuccinate after both regimens. After single and multiple doses, the respective areas under the curve of erythromycin base generated by estolate formulation were 3 and 1.6 times greater (P less than 0.05) than those of ethylsuccinate. The lower percentage of hydrolysis of erythromycin estolate (41 versus 69%) combined with its longer half-life (5.47 versus 2.72 h) and its larger area under the curve (30.61 versus 4.68 micrograms/h/ml, after multiple doses) could explain these differences. This study underscores the need for a specific high-pressure liquid chromatography assay and the importance of wide variability, rate-limited processes, changes with multiple doses, and the appearance of a second peak when one studies the pharmacokinetics of erythromycin esters. The pharmacokinetic data presented in this study reinforce the clinical advantages of erythromycin estolate over erythromycin ethylsuccinate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin K. L., Mather L. E., Philpot C. R., McDonald P. J. Intersubject and dose-related variability after intravenous administration of erythromycin. Br J Clin Pharmacol. 1980 Sep;10(3):273–279. doi: 10.1111/j.1365-2125.1980.tb01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Hamman J. W., Grundy W. E. Micromethod for assaying serum levels of erythromycin. Appl Microbiol. 1969 Jan;17(1):88–92. doi: 10.1128/am.17.1.88-92.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan T., Engeset A., Olszewski W., Josefsson K., Larsen N. Penetration of erythromycin into human peripheral lymph. J Antimicrob Chemother. 1982 Oct;10(4):319–324. doi: 10.1093/jac/10.4.319. [DOI] [PubMed] [Google Scholar]
- Chelvan P., Hamilton-Miller J. M., Brumfitt W. Biliary excretion of erythromycin after parenteral administration. Br J Clin Pharmacol. 1979 Sep;8(3):233–235. doi: 10.1111/j.1365-2125.1979.tb01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau D., Vallée F., Bergeron M. G., LeBel M. High-performance liquid chromatographic assay of erythromycin and its esters using electrochemical detection. J Chromatogr. 1987 Aug 7;419:205–212. doi: 10.1016/0378-4347(87)80278-5. [DOI] [PubMed] [Google Scholar]
- Derrick C. W., Dillon H. C., Jr Streptococcal pharyngitis therapy. A comparison of two erythromycin formulations. Am J Dis Child. 1979 Nov;133(11):1146–1148. doi: 10.1001/archpedi.1979.02130110054008. [DOI] [PubMed] [Google Scholar]
- Fraser D. G. Selection of an oral erythromycin product. Am J Hosp Pharm. 1980 Sep;37(9):1199–1205. [PubMed] [Google Scholar]
- Ginsburg C. M., McCracken G. H., Jr, Crow S. D., Dildy B. R., Morchower G., Steinberg J. B., Lancaster K. Erythromycin therapy for group A streptococcal pharyngitis. Results of a comparative study of the estolate and ethylsuccinate formulations. Am J Dis Child. 1984 Jun;138(6):536–539. doi: 10.1001/archpedi.1984.02140440020004. [DOI] [PubMed] [Google Scholar]
- Ginsburg C. M., McCracken G. H., Jr, Steinberg J. B., Crow S. D., Dildy B. F., Lancaster K., Olsen K. Management of group A streptococcal pharyngitis: a randomized controlled study of twice-daily erythromycin ethylsuccinate versus erythromycin estolate. Pediatr Infect Dis. 1982 Nov-Dec;1(6):384–387. [PubMed] [Google Scholar]
- Graffner C., Josefsson K., Stockman O. Intra- and intersubject variation of erythromycin absorption from single-unit and multiple-unit enteric-coated products. Biopharm Drug Dispos. 1986 Mar-Apr;7(2):163–171. doi: 10.1002/bdd.2510070207. [DOI] [PubMed] [Google Scholar]
- HAMMOND J. B., GRIFFITH R. S. Factors affecting the absorption and biliary excretion of erythromycin and two of its derivatives in humans. Clin Pharmacol Ther. 1961 May-Jun;2:308–312. doi: 10.1002/cpt196123308. [DOI] [PubMed] [Google Scholar]
- Hall K. W., Nightingale C. H., Gibaldi M., Nelson E., Bates T. R., DiSanto A. R. Pharmacokinetics of erythromycin in normal and alcoholic liver disease subjects. J Clin Pharmacol. 1982 Jul;22(7):321–325. doi: 10.1002/j.1552-4604.1982.tb02682.x. [DOI] [PubMed] [Google Scholar]
- Hovi T., Heikinheimo M. Effect of concomitant food intake on absorption kinetics of erythromycin in healthy volunteers. Eur J Clin Pharmacol. 1985;28(2):231–233. doi: 10.1007/BF00609699. [DOI] [PubMed] [Google Scholar]
- Josefsson K., Bergan T., Magni L. Dose-related pharmacokinetics after oral administration of a new formulation of erythromycin base. Br J Clin Pharmacol. 1982 May;13(5):685–691. doi: 10.1111/j.1365-2125.1982.tb01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson K., Steinbakk M., Bergan T., Midtvedt T., Magni L. Pharmacokinetics of a new, microencapsulated erythromycin base after repeated oral doses. Chemotherapy. 1982;28(3):176–184. doi: 10.1159/000238073. [DOI] [PubMed] [Google Scholar]
- Kucers A. Chloramphenicol, erythromycin, vancomycin, tetracyclines. Lancet. 1982 Aug 21;2(8295):425–429. doi: 10.1016/s0140-6736(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Le Blanc P. P., Dumas J. Calcul des valeurs initiales des paramètres pharmacocinétiques à l'aide d'un calculateur programmable. Therapie. 1983 Jan-Feb;38(1):21–26. [PubMed] [Google Scholar]
- Malmborg A. S. Effect of food on absorption of erythromycin. A study of two derivatives, the stearate and the base. J Antimicrob Chemother. 1979 Sep;5(5):591–599. doi: 10.1093/jac/5.5.591. [DOI] [PubMed] [Google Scholar]
- Mather L. E., Austin K. L., Philpot C. R., McDonald P. J. Absorption and bioavailability of oral erythromycin. Br J Clin Pharmacol. 1981 Aug;12(2):131–140. doi: 10.1111/j.1365-2125.1981.tb01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang K. S. A review of metabolite kinetics. J Pharmacokinet Biopharm. 1985 Dec;13(6):633–662. doi: 10.1007/BF01058905. [DOI] [PubMed] [Google Scholar]
- Perrier D., Mayersohn M. Noncompartmental determination of the steady-state volume of distribution for any mode of administration. J Pharm Sci. 1982 Mar;71(3):372–373. doi: 10.1002/jps.2600710332. [DOI] [PubMed] [Google Scholar]
- Shepard T. A., Reuning R. H., Aarons L. J. Interpretation of area under the curve measurements for drugs subject to enterohepatic cycling. J Pharm Sci. 1985 Feb;74(2):227–228. doi: 10.1002/jps.2600740228. [DOI] [PubMed] [Google Scholar]
- Smith I. L., Schentag J. J. Noncompartmental determination of the steady-state volume of distribution during multiple dosing. J Pharm Sci. 1984 Feb;73(2):281–282. doi: 10.1002/jps.2600730239. [DOI] [PubMed] [Google Scholar]
- Tardrew P. L., Mao J. C., Kenney D. Antibacterial activity of 2'-esters of erythromycin. Appl Microbiol. 1969 Aug;18(2):159–165. doi: 10.1128/am.18.2.159-165.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P. J., Burgess K. R., Marlin G. E. Influence of food on absorption of erythromycin ethyl succinate. Antimicrob Agents Chemother. 1980 Nov;18(5):829–831. doi: 10.1128/aac.18.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandramaga T. B., Van Hecken A., Mullie A., Verbesselt R., De Schepper P. J., Verbist L., Josefsson K. Relative bioavailability of enteric coated pellets, stearate and ethylsuccinate formulations of erythromycin. Pharmacology. 1984;29(6):305–311. doi: 10.1159/000138029. [DOI] [PubMed] [Google Scholar]
- Tserng K. Y., Wagner J. G. Fluorometric determination of erythromycin and erythromycin propionate in whole blood or plasma and correlation of results with microbiological assay. Anal Chem. 1976 Feb;48(2):348–353. doi: 10.1021/ac60366a033. [DOI] [PubMed] [Google Scholar]
- Welling P. G., Elliott R. L., Pitterle M. E., Corrick-West H. P., Lyons L. L. Plasma levels following single and repeated doses of erythromycin estolate and erythromycin stearate. J Pharm Sci. 1979 Feb;68(2):150–155. doi: 10.1002/jps.2600680208. [DOI] [PubMed] [Google Scholar]


